Draw The Shape Of D Orbital
News Leon
Mar 24, 2025 · 6 min read
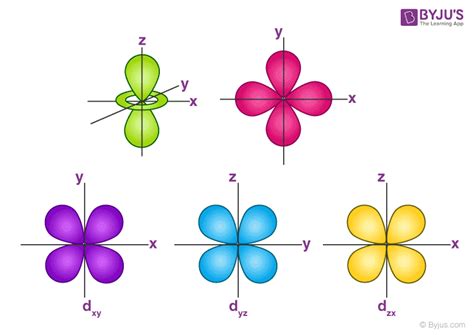
Table of Contents
Drawing the Shape of d Orbitals: A Comprehensive Guide
The world of atomic orbitals can seem daunting, especially when visualizing the complex shapes of d orbitals. Unlike the simple spherical s orbitals and dumbbell-shaped p orbitals, d orbitals exhibit a greater degree of complexity, with four of the five having a cloverleaf-like structure and the fifth possessing a unique dumbbell shape with a donut around the middle. Understanding their shapes is crucial to grasping concepts in chemistry, including bonding, molecular geometry, and spectroscopy. This comprehensive guide will delve into the intricacies of drawing d orbitals, explaining the underlying principles and providing step-by-step instructions.
Understanding the Quantum Numbers
Before we embark on drawing d orbitals, it's essential to understand the quantum numbers that define them. These numbers – principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms) – provide a complete description of an electron's state within an atom.
-
Principal Quantum Number (n): This number determines the energy level and size of the orbital. For d orbitals, n is always ≥ 3. This means d orbitals are found in the third energy level and beyond (3d, 4d, 5d, etc.).
-
Azimuthal Quantum Number (l): This number specifies the shape of the orbital. For d orbitals, l = 2. This value dictates the characteristic cloverleaf and dumbbell shapes.
-
Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. For d orbitals, ml can have five values: -2, -1, 0, +1, +2. This corresponds to the five different d orbitals within a subshell.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, either +1/2 or -1/2 (spin up or spin down). This is not directly related to the orbital's shape, but is important for understanding electron configurations and Hund's rule.
The Five d Orbitals: A Detailed Look
The five d orbitals, denoted as d<sub>xy</sub>, d<sub>xz</sub>, d<sub>yz</sub>, d<sub>x²−y²</sub>, and d<sub>z²</sub>, are distinguished by their spatial orientations and nodal planes (regions of zero electron density). Let’s break down each one:
1. d<sub>xy</sub> Orbital
The d<sub>xy</sub> orbital has four lobes arranged in a cloverleaf pattern within the xy-plane. The lobes lie between the x and y axes, extending along the bisectors of the axes. It has two nodal planes: the xz-plane and the yz-plane. Think of it like a four-leaf clover lying flat on the xy-plane.
Drawing the d<sub>xy</sub> orbital:
- Draw the x and y axes.
- Draw four lobes, two in the positive and two in the negative quadrants of the xy-plane.
- Ensure the lobes are roughly equal in size and point between the axes.
- Indicate the nodal planes (xz and yz planes) with dashed lines or shading.
2. d<sub>xz</sub> Orbital
Similar to the d<sub>xy</sub> orbital, the d<sub>xz</sub> orbital also possesses a cloverleaf shape, but its lobes lie in the xz-plane, situated between the x and z axes. Its nodal planes are the xy and yz planes.
Drawing the d<sub>xz</sub> orbital:
- Draw the x and z axes.
- Draw four lobes, with two extending into the positive and two into the negative regions of the xz-plane, between the axes.
- Indicate the nodal planes (xy and yz planes) with dashed lines or shading.
3. d<sub>yz</sub> Orbital
The d<sub>yz</sub> orbital is analogous to the d<sub>xz</sub> and d<sub>xy</sub> orbitals, with its cloverleaf structure located in the yz-plane, between the y and z axes. The nodal planes are the xy and xz planes.
Drawing the d<sub>yz</sub> orbital:
- Draw the y and z axes.
- Draw four lobes, with two extending into the positive and two into the negative regions of the yz-plane, between the axes.
- Indicate the nodal planes (xy and xz planes) with dashed lines or shading.
4. d<sub>x²−y²</sub> Orbital
The d<sub>x²−y²</sub> orbital differs slightly from the previous three. It still has four lobes, but these lobes point directly along the x and y axes. It has two nodal planes bisecting the x and y axes at 45-degree angles.
Drawing the d<sub>x²−y²</sub> orbital:
- Draw the x and y axes.
- Draw four lobes, two along the positive x-axis and two along the positive and negative y-axes.
- Indicate the nodal planes (bisecting the axes at 45 degrees) with dashed lines or shading.
5. d<sub>z²</sub> Orbital
The d<sub>z²</sub> orbital is unique. It has a dumbbell shape along the z-axis, with a torus (donut) around the center in the xy-plane. It has two nodal planes, which are conical in shape and extend from the xy-plane.
Drawing the d<sub>z²</sub> orbital:
- Draw the z-axis.
- Draw a dumbbell shape along the z-axis, with lobes at the positive and negative ends.
- Draw a torus (donut) around the center in the xy-plane.
- Indicate the conical nodal planes with shading or dashed lines.
Tips for Accurate Drawing
Creating accurate representations of d orbitals requires practice and attention to detail. Here are some tips to enhance your drawings:
- Use a consistent scale: Ensure the lobes of each orbital are proportionally sized.
- Clearly indicate nodal planes: Use dashed lines or shading to delineate regions of zero electron density.
- Label the orbitals: Always label each orbital (d<sub>xy</sub>, d<sub>xz</sub>, etc.) for clarity.
- Use 3D representation: While 2D drawings are necessary, try to visualize the orbitals in three dimensions. Consider using different shading or perspective techniques to enhance depth.
- Practice, practice, practice: The more you practice drawing d orbitals, the more comfortable and accurate you will become.
Applications of Understanding d Orbital Shapes
Understanding the shapes of d orbitals is crucial for various applications in chemistry:
- Crystal Field Theory: The shapes and orientations of d orbitals are fundamental to understanding how ligands interact with transition metal ions in coordination complexes, explaining their magnetic properties and colors.
- Molecular Orbital Theory: The d orbitals play a significant role in forming molecular orbitals in transition metal complexes, influencing their bonding and reactivity.
- Spectroscopy: Transitions between different d orbitals are responsible for the characteristic absorption and emission spectra of transition metal complexes, leading to their vibrant colors.
- Catalysis: The unique shapes and electronic properties of d orbitals are essential to the catalytic activity of transition metal complexes in numerous industrial processes.
Conclusion
Mastering the art of drawing d orbitals is a journey that requires understanding quantum numbers and the spatial arrangement of electron density. By following the step-by-step instructions and incorporating the helpful tips provided, you can accurately represent these complex orbitals. This understanding will significantly enhance your comprehension of various advanced chemical concepts and their practical applications. Remember, consistent practice is key to achieving proficiency. Keep drawing, and you'll find yourself visualizing and understanding d orbitals with increasing ease and accuracy. The effort invested in mastering this skill will significantly contribute to a deeper appreciation of the beauty and complexity of the atomic world.
Latest Posts
Latest Posts
-
Anything That Has Mass And Takes Up Space Is Called
Mar 26, 2025
-
How Many Protons And Electrons In Magnesium
Mar 26, 2025
-
Which Nitrogenous Base Is Not Found In Rna
Mar 26, 2025
-
A Particle Moves Horizontally In Uniform Circular Motion
Mar 26, 2025
-
The Resistivity Of A Wire Depends On
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Draw The Shape Of D Orbital . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
