Draw The Major Product Formed In The Reaction.
News Leon
Mar 23, 2025 · 6 min read
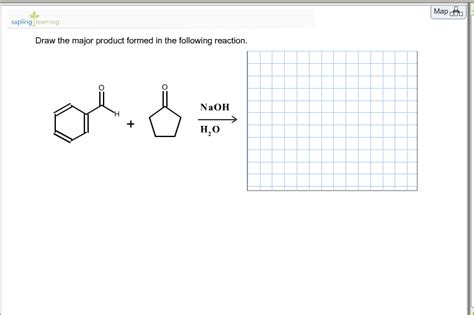
Table of Contents
Drawing the Major Product Formed in a Reaction: A Comprehensive Guide
Predicting the major product of an organic reaction is a cornerstone of organic chemistry. It requires a deep understanding of reaction mechanisms, reaction kinetics, and the inherent stability of different functional groups and intermediates. This comprehensive guide will equip you with the tools and knowledge to successfully tackle this crucial aspect of organic chemistry. We'll delve into various reaction types, highlighting key considerations for determining the major product. We'll also cover several helpful strategies and tips to improve your predictive capabilities.
Understanding Reaction Mechanisms: The Foundation of Prediction
Before predicting the major product, understanding the reaction mechanism is paramount. The mechanism dictates the step-by-step process of bond breaking and bond formation, ultimately leading to the product(s). Different mechanisms lead to different products, even with the same starting materials and reagents.
Key mechanistic concepts to consider:
-
Nucleophilic attack: Understanding which atom acts as a nucleophile (electron-rich species) and which acts as an electrophile (electron-deficient species) is crucial. Nucleophiles attack electrophiles, leading to bond formation.
-
Leaving groups: The ability of a group to leave as a stable anion influences the reaction's outcome. Good leaving groups stabilize the negative charge more effectively, leading to faster reactions and cleaner product formation. Examples include halides (Cl⁻, Br⁻, I⁻), tosylates, and mesylates.
-
Carbocation stability: In reactions involving carbocations (positively charged carbon atoms), the stability of the carbocation intermediate significantly affects the product distribution. Tertiary carbocations are more stable than secondary, which are more stable than primary. This stability is due to hyperconjugation and inductive effects.
-
Stereochemistry: Consider stereochemistry (3D arrangement of atoms) throughout the reaction mechanism. Reactions can proceed with retention, inversion, or racemization of stereochemistry. This is particularly important in reactions involving chiral centers.
-
Thermodynamics and Kinetics: The most stable product isn't always the major product. Kinetics (reaction rates) can sometimes outweigh thermodynamics (relative stability of products). A faster reaction, even if it leads to a less stable product, might dominate, especially at lower temperatures.
Predicting Major Products in Different Reaction Types
Let's explore some common reaction types and the key factors influencing the major product formed:
1. SN1 and SN2 Reactions
-
SN1 (Substitution Nucleophilic Unimolecular): This reaction proceeds through a carbocation intermediate. The rate-determining step is the formation of the carbocation. Therefore, the stability of the carbocation dictates the major product. More substituted carbocations (tertiary > secondary > primary) are more stable and lead to the major product. SN1 reactions often lead to racemization at the chiral center.
-
SN2 (Substitution Nucleophilic Bimolecular): This reaction proceeds in a single step, with the nucleophile attacking the substrate from the backside, leading to inversion of configuration at the chiral center. Steric hindrance around the electrophilic carbon significantly impacts the reaction rate. Primary halides react faster than secondary, which react faster than tertiary (due to steric effects).
2. Elimination Reactions (E1 and E2)
-
E1 (Elimination Unimolecular): Similar to SN1, E1 reactions proceed through a carbocation intermediate. The major product is usually the more substituted alkene (Zaitsev's rule), due to the greater stability of the alkene.
-
E2 (Elimination Bimolecular): E2 reactions occur in a single step, with the base abstracting a proton and the leaving group departing simultaneously. The stereochemistry of the starting material plays a significant role. Anti-periplanar geometry (180° dihedral angle between the proton and the leaving group) is favored for E2 reactions. Zaitsev's rule also often applies to E2 reactions, predicting the more substituted alkene as the major product. However, steric factors and the strength and nature of the base can influence the product distribution.
3. Addition Reactions
-
Electrophilic Addition: These reactions involve the addition of an electrophile to a double or triple bond. Markovnikov's rule often predicts the major product in electrophilic additions to unsymmetrical alkenes. The electrophile adds to the carbon atom with more hydrogen atoms, leading to the more stable carbocation intermediate.
-
Nucleophilic Addition: In these reactions, a nucleophile adds to a carbonyl group (C=O). The addition often leads to a tetrahedral intermediate, which can subsequently undergo various transformations, depending on the reaction conditions.
4. Grignard Reactions
Grignard reagents (RMgX) are strong nucleophiles that react with carbonyl compounds. The addition of a Grignard reagent to a carbonyl group results in the formation of an alkoxide intermediate, which is then protonated to give an alcohol. The reaction is highly regioselective and stereoselective.
Strategies for Predicting Major Products
-
Draw out the complete reaction mechanism: This allows you to visualize each step, identify intermediates, and understand the factors influencing the product distribution.
-
Consider all possible products: Even though you're aiming for the major product, considering all possible products can help you understand the reaction better and refine your prediction.
-
Analyze the reaction conditions: Temperature, solvent, and the strength and nature of the reagents significantly influence the reaction pathway and product distribution.
-
Apply relevant rules: Utilize rules such as Markovnikov's rule, Zaitsev's rule, and considerations of carbocation stability to refine your predictions.
-
Practice, practice, practice: The more you practice drawing reaction mechanisms and predicting products, the better you'll become at it.
Examples: Predicting Major Products
Let's consider a few examples to illustrate these principles:
Example 1: SN1 Reaction
The reaction of tert-butyl bromide with methanol in the presence of a weak acid would primarily yield tert-butyl methyl ether. This is because the tertiary carbocation intermediate formed is highly stable, leading to a fast SN1 reaction.
Example 2: SN2 Reaction
The reaction of methyl bromide with sodium cyanide (NaCN) would predominantly yield acetonitrile. This is an SN2 reaction, and the primary halide reacts readily with the strong nucleophile.
Example 3: E1 Reaction
The reaction of 2-bromo-2-methylpropane with ethanol under acidic conditions would result in the formation of 2-methylpropene as the major product (Zaitsev's rule).
Example 4: E2 Reaction
The reaction of 2-bromobutane with a strong base like potassium tert-butoxide (t-BuOK) would yield predominantly 2-butene (Zaitsev's product). The strong base promotes the elimination reaction, and the more substituted alkene is favored.
Advanced Considerations: Beyond Simple Reactions
Many reactions are more complex and involve multiple steps or competing pathways. Understanding the relative rates of these competing pathways is essential for accurately predicting the major product. These advanced scenarios often require a deeper understanding of:
-
Concerted reactions: Reactions where bond breaking and bond formation occur simultaneously.
-
Pericyclic reactions: Reactions involving cyclic transition states, such as Diels-Alder reactions.
-
Radical reactions: Reactions involving free radicals as intermediates.
Conclusion
Predicting the major product formed in a reaction is a challenging but rewarding skill in organic chemistry. By systematically analyzing the reaction mechanism, considering the stability of intermediates, applying relevant rules, and practicing diligently, you can significantly enhance your ability to accurately predict the major product and gain a deeper understanding of organic chemistry. Remember to carefully consider the reaction conditions and the nature of the reagents, as these factors play a crucial role in determining the reaction pathway and the final product formed. Continuous learning and practice are key to mastering this important skill.
Latest Posts
Latest Posts
-
Which Poem Are These Lines From
Mar 25, 2025
-
What Is Not A Subatomic Particle
Mar 25, 2025
-
All Populations Of All Species In A Given Area
Mar 25, 2025
-
Zn Hcl Zncl2 H2 Balance Equation
Mar 25, 2025
-
What Is The Unit Of Entropy
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Product Formed In The Reaction. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
