What Is The Unit Of Entropy
News Leon
Mar 25, 2025 · 5 min read
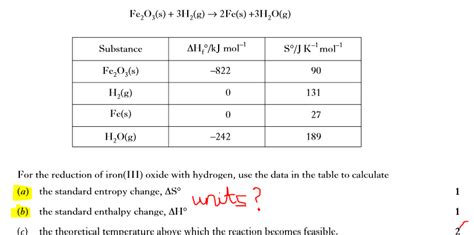
Table of Contents
- What Is The Unit Of Entropy
- Table of Contents
- What is the Unit of Entropy? A Deep Dive into Thermodynamics and Information Theory
- Understanding Entropy: A Multifaceted Concept
- The Unit of Entropy: Joules per Kelvin (J/K)
- Why Kelvin?
- Entropy in Information Theory: Bits and Nats
- Connecting Thermodynamic and Information Entropy: A Bridge Between Disciplines
- Misconceptions about Entropy Units
- Beyond the Basics: Entropy in Advanced Applications
- Conclusion: Mastering the Units of Entropy
- Latest Posts
- Latest Posts
- Related Post
What is the Unit of Entropy? A Deep Dive into Thermodynamics and Information Theory
Entropy, a cornerstone concept in both thermodynamics and information theory, measures the degree of disorder or randomness within a system. Understanding its unit is crucial to grasping its implications across various scientific disciplines. This article delves deep into the definition of entropy, explores its units in different contexts, and clarifies common misconceptions.
Understanding Entropy: A Multifaceted Concept
Before we dive into the units, let's solidify our understanding of entropy itself. Entropy isn't simply "disorder"; it's a precise measure of the number of possible microscopic states that correspond to a given macroscopic state. A highly ordered system, like a neatly stacked deck of cards, has low entropy because there's only one arrangement that corresponds to that order. A shuffled deck, however, has high entropy because countless arrangements produce a seemingly random order.
This concept applies across diverse fields:
-
Thermodynamics: In thermodynamics, entropy quantifies the amount of thermal energy unavailable for doing useful work in a thermodynamic process at a given temperature. An increase in entropy signifies an irreversible process, where energy is dispersed and becomes less usable.
-
Information Theory: In information theory, entropy measures the uncertainty or information content of a message or random variable. A highly predictable message has low entropy, while a completely random message has high entropy. This connection between thermodynamic entropy and information entropy is profound and highlights the fundamental link between physics and information.
The Unit of Entropy: Joules per Kelvin (J/K)
In thermodynamics, the standard unit of entropy is joules per kelvin (J/K). This stems directly from the fundamental thermodynamic relationship:
ΔS = Qrev/T
Where:
- ΔS represents the change in entropy.
- Qrev is the heat added or removed reversibly (meaning infinitely slowly).
- T is the absolute temperature in Kelvin.
This equation shows that entropy change is proportional to the reversible heat transfer divided by the absolute temperature. Since heat is measured in joules (J) and temperature in kelvin (K), the resulting unit for entropy change is J/K. It's important to remember that this unit applies to the change in entropy (ΔS), not the absolute entropy itself. Calculating absolute entropy requires a more complex approach.
Why Kelvin?
The use of Kelvin (the absolute temperature scale) is crucial. The Kelvin scale starts at absolute zero (0 K), the theoretical point where all molecular motion ceases. Using Celsius or Fahrenheit would introduce inconsistencies and inaccuracies because these scales have arbitrary zero points.
Entropy in Information Theory: Bits and Nats
In information theory, the unit of entropy depends on the base of the logarithm used in the entropy calculation. The most common units are:
-
Bits: Used when the logarithm base is 2. One bit represents the amount of information gained when the uncertainty about a binary event (e.g., a coin flip) is resolved.
-
Nats: Used when the logarithm base is e (the natural logarithm). One nat represents the amount of information gained when the uncertainty about a continuous variable is reduced by a factor of e.
The choice between bits and nats depends on the context. Bits are prevalent in digital communication and computer science, while nats are often favored in mathematical derivations and statistical mechanics due to the natural logarithm's mathematical properties. The conversion between bits and nats is straightforward: 1 nat = ln(2) bits ≈ 0.693 bits.
Connecting Thermodynamic and Information Entropy: A Bridge Between Disciplines
While seemingly disparate, thermodynamic and information entropy share a deep connection. This connection is often explored through Boltzmann's constant (k<sub>B</sub>), which acts as a conversion factor between the two systems:
S = k<sub>B</sub> ln(W)
Where:
- S is thermodynamic entropy.
- k<sub>B</sub> is Boltzmann's constant (approximately 1.38 × 10<sup>-23</sup> J/K).
- W is the number of microscopic states corresponding to the macroscopic state.
This equation, known as Boltzmann's entropy formula, bridges the gap. It shows that the thermodynamic entropy is proportional to the logarithm of the number of possible microstates, directly relating thermodynamic entropy (measured in J/K) to the information-theoretic concept of the number of possible states.
Misconceptions about Entropy Units
Several misconceptions surround the units of entropy, especially when bridging thermodynamics and information theory:
-
Mixing units: It's incorrect to directly compare entropy values expressed in J/K and bits or nats without appropriate conversion using Boltzmann's constant. These are distinct units measuring related but different aspects of entropy.
-
Absolute vs. change: The J/K unit applies to changes in entropy (ΔS). Absolute entropy values (S) require a reference point and careful calculation using statistical mechanics.
-
Entropy as a substance: Entropy is not a substance that can be contained or transferred like energy or mass. It's a state function describing the system's disorder or information content.
Beyond the Basics: Entropy in Advanced Applications
The J/K and bit/nat units provide a foundation for understanding entropy in various advanced applications:
-
Statistical Mechanics: Entropy plays a central role in statistical mechanics, providing a link between microscopic properties of a system and its macroscopic behavior. Understanding entropy units is fundamental to interpreting statistical mechanical calculations.
-
Black Hole Thermodynamics: The concept of entropy extends to black holes, where the entropy of a black hole is proportional to its surface area. This has profound implications for our understanding of gravity and thermodynamics.
-
Cosmology: Entropy plays a key role in the evolution of the universe, with the universe exhibiting a constant increase in entropy over time.
Conclusion: Mastering the Units of Entropy
The unit of entropy, whether J/K in thermodynamics or bits/nats in information theory, reflects its fundamental role in describing disorder and information. Understanding these units, their connections, and potential pitfalls is crucial for anyone working with entropy across diverse scientific and engineering disciplines. A firm grasp of these concepts is vital for accurate interpretations and a deeper understanding of the universe's fundamental laws. Remember that the appropriate choice of units depends heavily on the specific application and context, making careful consideration essential for avoiding confusion and maintaining accuracy in calculations and analyses. The connection between the thermodynamic and information-theoretic interpretations of entropy highlights a unifying principle in science, reinforcing the power of entropy as a fundamental concept across seemingly disparate fields.
Latest Posts
Latest Posts
-
In The Figure Two Large Thin Metal Plates
Mar 28, 2025
-
How Many Feet In 0 4 Miles
Mar 28, 2025
-
The Rate Of A Chemical Reaction Depends On
Mar 28, 2025
-
What Do You Call People From France
Mar 28, 2025
-
Match The Label To The Correct Structure On The Chloroplast
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Unit Of Entropy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
