Zn Hcl Zncl2 H2 Balance Equation
News Leon
Mar 25, 2025 · 6 min read
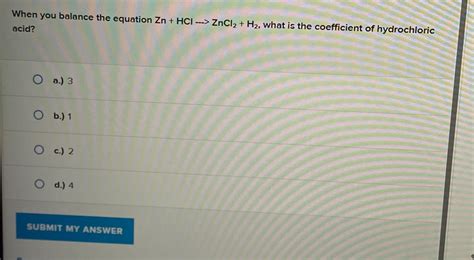
Table of Contents
Zn + HCl → ZnCl₂ + H₂: A Deep Dive into the Balanced Equation and its Implications
The seemingly simple chemical reaction between zinc (Zn) and hydrochloric acid (HCl) to produce zinc chloride (ZnCl₂) and hydrogen gas (H₂) is a rich example of a single displacement reaction, offering a gateway to understanding fundamental concepts in chemistry. This article delves deep into this reaction, exploring its balanced equation, the stoichiometry involved, the reaction mechanism, and its practical applications. We'll also discuss safety precautions and considerations when working with these chemicals.
Understanding the Reaction: Zn + HCl → ZnCl₂ + H₂
The reaction between zinc metal and hydrochloric acid is a classic example of a single displacement reaction, also known as a single replacement reaction. In this type of reaction, a more reactive element displaces a less reactive element from its compound. In this case, zinc, being more reactive than hydrogen, displaces hydrogen from hydrochloric acid.
The unbalanced equation for this reaction is:
Zn + HCl → ZnCl₂ + H₂
This equation, however, doesn't reflect the law of conservation of mass. To balance the equation, we need to ensure the number of atoms of each element is the same on both sides of the equation.
The Balanced Equation:
The balanced equation for the reaction is:
Zn + 2HCl → ZnCl₂ + H₂
This balanced equation shows that one mole of zinc reacts with two moles of hydrochloric acid to produce one mole of zinc chloride and one mole of hydrogen gas. This ratio is crucial for understanding the stoichiometry of the reaction and performing calculations involving reactant and product quantities.
Stoichiometry and Calculations
Stoichiometry is the section of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. The balanced equation provides the foundation for stoichiometric calculations. Let's consider some examples:
Example 1: Determining the amount of hydrogen gas produced.
If we react 65.38 grams of zinc (one mole) with excess hydrochloric acid, how many grams of hydrogen gas will be produced?
-
Moles of Zinc: The molar mass of zinc is approximately 65.38 g/mol. Therefore, 65.38 g of zinc represents 1 mole of zinc.
-
Moles of Hydrogen: According to the balanced equation, 1 mole of zinc produces 1 mole of hydrogen gas.
-
Grams of Hydrogen: The molar mass of hydrogen gas (H₂) is approximately 2.02 g/mol. Therefore, 1 mole of hydrogen gas weighs 2.02 g.
Conclusion: 2.02 grams of hydrogen gas will be produced.
Example 2: Determining the amount of HCl needed.
How many grams of hydrochloric acid are needed to completely react with 130.76 g of zinc?
-
Moles of Zinc: 130.76 g of zinc is equivalent to 2 moles of zinc (130.76 g / 65.38 g/mol = 2 mol).
-
Moles of HCl: The balanced equation shows that 2 moles of HCl are required for every 1 mole of zinc. Therefore, 4 moles of HCl are needed (2 mol Zn * 2 mol HCl/mol Zn = 4 mol HCl).
-
Grams of HCl: The molar mass of HCl is approximately 36.46 g/mol. Therefore, 4 moles of HCl weigh 145.84 g (4 mol * 36.46 g/mol = 145.84 g).
Conclusion: 145.84 grams of hydrochloric acid are needed to completely react with 130.76 g of zinc.
These examples demonstrate the power of the balanced equation in performing quantitative analysis of chemical reactions. Understanding stoichiometry is critical for various applications in chemistry, including industrial processes and laboratory experiments.
The Reaction Mechanism
The reaction between zinc and hydrochloric acid proceeds through a series of steps involving electron transfer. This is an example of a redox reaction (reduction-oxidation reaction):
-
Oxidation of Zinc: Zinc atoms lose two electrons to become zinc ions (Zn²⁺). This is an oxidation process because zinc loses electrons.
Zn → Zn²⁺ + 2e⁻
-
Reduction of Hydrogen Ions: Hydrogen ions (H⁺) from the hydrochloric acid gain electrons to form hydrogen gas (H₂). This is a reduction process because hydrogen gains electrons.
2H⁺ + 2e⁻ → H₂
The overall reaction is the sum of these two half-reactions. The electrons released during the oxidation of zinc are consumed during the reduction of hydrogen ions. This electron transfer is the driving force behind the reaction.
Practical Applications
The reaction between zinc and hydrochloric acid has several practical applications:
-
Hydrogen Gas Production: This reaction is a common method for producing hydrogen gas in the laboratory. Hydrogen is a valuable chemical used in various industries, including fuel cells and ammonia production.
-
Cleaning and Etching: The reaction can be used to clean and etch metal surfaces. The etching process is based on the reaction dissolving a thin layer of metal. This is commonly applied in metal work or preparing surfaces for further treatment.
-
Production of Zinc Chloride: Zinc chloride is an important industrial chemical with various uses, including as a wood preservative, soldering flux, and catalyst.
-
Educational Purposes: The reaction is widely used in educational settings to illustrate fundamental concepts in chemistry, such as stoichiometry, redox reactions, and gas evolution.
Safety Precautions
It's crucial to handle chemicals involved in this reaction with care due to potential hazards:
-
Hydrochloric Acid: Hydrochloric acid is a corrosive acid that can cause severe burns to the skin and eyes. Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat, when handling this chemical.
-
Hydrogen Gas: Hydrogen gas is flammable and can form explosive mixtures with air. Ensure adequate ventilation and avoid any open flames or sparks when working with hydrogen gas.
-
Proper Disposal: Dispose of the reaction products according to local regulations. Never pour chemicals down the drain without prior approval from relevant authorities.
Following these safety guidelines is essential to prevent accidents and injuries during the experiment.
Further Considerations and Related Reactions
This reaction can be influenced by several factors, including:
-
Concentration of HCl: A higher concentration of HCl will generally lead to a faster reaction rate.
-
Surface Area of Zinc: A larger surface area of zinc (e.g., using zinc powder instead of a zinc block) will also increase the reaction rate.
-
Temperature: Increasing the temperature typically accelerates the reaction rate.
The reaction between zinc and other acids, such as sulfuric acid (H₂SO₄) and nitric acid (HNO₃), are similar, resulting in the formation of the corresponding zinc salt and hydrogen gas (with nitric acid, the reaction is more complex and can produce other products as well). However, the reaction rate and the exact reaction products may vary depending on the specific acid used.
Conclusion
The reaction between zinc and hydrochloric acid, represented by the balanced equation Zn + 2HCl → ZnCl₂ + H₂, is a fundamental chemical reaction with significant implications in chemistry and various industrial processes. Understanding the balanced equation, stoichiometry, reaction mechanism, and safety precautions associated with this reaction is crucial for anyone working with these chemicals. This reaction provides a valuable platform for illustrating basic chemical concepts and their real-world applications. Remember always to prioritize safety and follow proper laboratory procedures when conducting experiments involving these chemicals.
Latest Posts
Latest Posts
-
Linear Mass Density Of A String
Mar 28, 2025
-
Which Type Of Substance Cannot Be Separated Physically
Mar 28, 2025
-
What Is The Transcribed Mrna Strand For Cattaa
Mar 28, 2025
-
What Is The Final Electron Acceptor In The Etc
Mar 28, 2025
-
The Purpose Of Cellular Respiration Is To
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Zn Hcl Zncl2 H2 Balance Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
