Does A Hydrogen Atom Have A Neutron
News Leon
Apr 02, 2025 · 5 min read
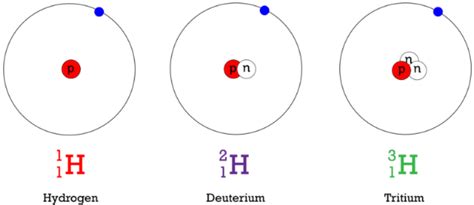
Table of Contents
- Does A Hydrogen Atom Have A Neutron
- Table of Contents
- Does a Hydrogen Atom Have a Neutron? Unveiling the Mysteries of the Simplest Atom
- Understanding the Basic Structure of an Atom
- The Case of Hydrogen: The Simplest Atom
- Isotopes: Variations on a Theme
- Answering the Question: Does a Hydrogen Atom Have a Neutron?
- The Significance of Isotopes in Various Fields
- Exploring the Nuclear Forces: Why Neutrons Matter
- Beyond the Basics: Delving Deeper into Isotopic Abundance and Applications
- Conclusion: A Comprehensive Look at Hydrogen's Isotopes
- Latest Posts
- Latest Posts
- Related Post
Does a Hydrogen Atom Have a Neutron? Unveiling the Mysteries of the Simplest Atom
The hydrogen atom, the most abundant element in the universe, often serves as the foundational building block for understanding atomic structure. Its simplicity, however, belies a fascinating complexity when we delve into its subatomic composition. A fundamental question that often arises is: Does a hydrogen atom have a neutron? The answer, while seemingly straightforward, provides a springboard for exploring the nuances of isotopes, nuclear stability, and the very nature of matter itself.
Understanding the Basic Structure of an Atom
Before we address the central question, let's establish a basic understanding of atomic structure. An atom comprises three fundamental subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's atomic number and its identity.
- Neutrons: Neutrally charged particles also located in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or orbitals. Their number typically equals the number of protons in a neutral atom.
The Case of Hydrogen: The Simplest Atom
Hydrogen, with an atomic number of 1, possesses only one proton in its nucleus. In its most common form, protium, it also has one electron orbiting this proton. This simplicity makes it an ideal starting point for understanding atomic behavior. However, the presence or absence of neutrons adds a layer of complexity.
Isotopes: Variations on a Theme
The existence of isotopes significantly impacts the answer to our central question. Isotopes are atoms of the same element (same number of protons) but with differing numbers of neutrons. This variation in neutron count leads to different mass numbers (the sum of protons and neutrons).
For hydrogen, we have three main isotopes:
- Protium (¹H): This is the most common isotope, containing one proton and no neutrons. Its mass number is 1.
- Deuterium (²H or D): Deuterium contains one proton and one neutron, giving it a mass number of 2. It's a stable isotope, meaning it doesn't readily decay.
- Tritium (³H or T): Tritium has one proton and two neutrons, resulting in a mass number of 3. It's a radioactive isotope, meaning it undergoes radioactive decay, transforming into helium-3.
Answering the Question: Does a Hydrogen Atom Have a Neutron?
The answer, therefore, is nuanced:
- A protium atom (the most common type of hydrogen atom) does not have a neutron.
- A deuterium atom (a stable isotope of hydrogen) does have one neutron.
- A tritium atom (a radioactive isotope of hydrogen) does have two neutrons.
Thus, the presence of a neutron in a hydrogen atom depends entirely on which isotope we're considering. The statement "a hydrogen atom has a neutron" is incomplete without specifying the isotope.
The Significance of Isotopes in Various Fields
The existence of hydrogen isotopes has far-reaching implications across various scientific and technological domains:
-
Nuclear Fusion: Deuterium and tritium are crucial in nuclear fusion reactions, a potential source of clean energy. The fusion of deuterium and tritium nuclei releases a significant amount of energy.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: Deuterium's unique nuclear spin properties make it valuable in NMR spectroscopy, a technique used for analyzing molecular structures. Deuterated solvents are frequently used to improve spectral resolution.
-
Tracers in Biological Research: Tritium's radioactivity makes it useful as a tracer in biological research. Scientists can track the movement and distribution of molecules within living organisms using tritium-labeled compounds.
-
Geochronology: The ratio of deuterium to protium in water samples can provide valuable information about past climates and geological processes.
Exploring the Nuclear Forces: Why Neutrons Matter
The presence of neutrons in the nucleus plays a vital role in nuclear stability. The strong nuclear force, which binds protons and neutrons together in the nucleus, is responsible for holding the atom together. However, the electrostatic repulsion between positively charged protons can disrupt this stability. Neutrons act as a kind of nuclear "glue," counteracting the repulsive forces between protons and stabilizing the nucleus, especially in heavier atoms.
In the case of hydrogen, the need for neutrons to stabilize the nucleus is less pronounced. Protium, with only a single proton, is perfectly stable without any neutrons. The addition of neutrons in deuterium and tritium, however, leads to slight changes in the atom's properties. Tritium’s instability highlights how the balance of nuclear forces can be altered by changes in neutron numbers.
Beyond the Basics: Delving Deeper into Isotopic Abundance and Applications
The relative abundance of hydrogen isotopes in nature also influences their importance. Protium accounts for the vast majority (over 99.98%) of naturally occurring hydrogen atoms. Deuterium constitutes a small but significant fraction, while tritium is present only in trace amounts due to its radioactive decay.
These variations in abundance have profound consequences for various applications. For example, the significantly higher abundance of protium makes it the primary form used in chemical reactions and industrial processes. On the other hand, the unique properties of deuterium and tritium are exploited in specialized applications such as nuclear fusion research and biological tracing experiments.
Conclusion: A Comprehensive Look at Hydrogen's Isotopes
In conclusion, the question of whether a hydrogen atom possesses a neutron depends on the specific isotope being discussed. While the most common isotope, protium, lacks a neutron, the stable deuterium isotope contains one, and the radioactive tritium isotope contains two. This seemingly simple difference has enormous consequences for various scientific and technological fields, from clean energy research to biological studies and beyond. Understanding the nuances of hydrogen isotopes is essential for comprehending the intricacies of atomic structure and the behavior of matter at the nuclear level. The seemingly simple hydrogen atom, therefore, presents a surprisingly complex and fascinating window into the wonders of the subatomic world. Further research into the properties and applications of different isotopes continues to unveil new insights and potential uses for these fundamental building blocks of our universe.
Latest Posts
Latest Posts
-
Is Strontium Hydroxide A Strong Base
Apr 05, 2025
-
Unsymmetrical Ethers Can Be Made By The Williamson Synthesis
Apr 05, 2025
-
Is Mitochondria Found In Plant Or Animal Cells
Apr 05, 2025
-
How Do You Draw A Cumulative Frequency Graph
Apr 05, 2025
-
200 Centimeters Equals How Many Meters
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Does A Hydrogen Atom Have A Neutron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
