Do Metals Form Anions Or Cations
News Leon
Mar 29, 2025 · 6 min read
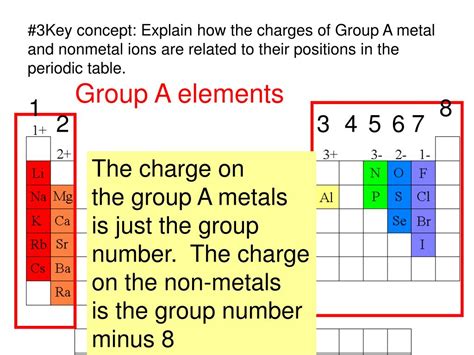
Table of Contents
Do Metals Form Anions or Cations? Understanding Metallic Properties and Ion Formation
The question of whether metals form anions or cations is fundamental to understanding chemistry. The answer, in short, is that metals overwhelmingly form cations, positively charged ions. This behavior is a defining characteristic of metals and is directly linked to their electronic structure and the nature of metallic bonding. This article delves deep into this topic, explaining the reasons behind this behavior, exploring exceptions, and examining the implications for various chemical processes.
The Electronic Structure of Metals: The Key to Cation Formation
Metals are characterized by their relatively low electronegativity and low ionization energies. Electronegativity measures an atom's ability to attract electrons towards itself in a chemical bond. Metals, having low electronegativity, have a weaker pull on electrons compared to non-metals. Ionization energy is the energy required to remove an electron from a neutral atom. Metals, with their low ionization energies, readily lose electrons.
This ease of electron loss is a consequence of their electronic structure. Metals typically have few valence electrons – the electrons in the outermost shell. These valence electrons are loosely held and are relatively far from the nucleus, experiencing a weaker electrostatic attraction. This makes them easily removed, resulting in the formation of a positively charged ion, or a cation.
Comparing Metals and Non-metals: A Contrast in Ion Formation
To further illustrate the tendency of metals to form cations, let's contrast their behavior with non-metals. Non-metals generally have high electronegativity and high ionization energies. They tend to attract electrons strongly and require significant energy to remove an electron. As a result, non-metals tend to gain electrons to achieve a stable electron configuration (often a full outermost shell), forming anions, negatively charged ions.
This difference in behavior is the foundation of ionic bonding, where the electrostatic attraction between positively charged metal cations and negatively charged non-metal anions holds the compound together. Examples include sodium chloride (NaCl), where sodium (Na) forms a Na⁺ cation and chlorine (Cl) forms a Cl⁻ anion, and magnesium oxide (MgO), where magnesium (Mg) forms a Mg²⁺ cation and oxygen (O) forms an O²⁻ anion.
The Formation of Cations: A Detailed Look
The process of cation formation involves the removal of one or more valence electrons from a metal atom. The number of electrons lost determines the charge of the cation. For instance:
- Group 1 metals (alkali metals): These metals have one valence electron and readily lose it to form a +1 cation (e.g., Na⁺, K⁺, Li⁺).
- Group 2 metals (alkaline earth metals): These metals have two valence electrons and lose both to form a +2 cation (e.g., Mg²⁺, Ca²⁺, Ba²⁺).
- Transition metals: Transition metals exhibit variable oxidation states, meaning they can lose different numbers of electrons to form cations with varying charges (e.g., Fe²⁺, Fe³⁺, Cu⁺, Cu²⁺). This variable behavior is due to the involvement of both s and d electrons in bonding.
The stability of the resulting cation is influenced by several factors, including the electron configuration of the ion and the effective nuclear charge. Cations with a noble gas electron configuration (a full outermost shell) are particularly stable.
Factors influencing the charge of a metal cation
The charge a metal ion carries is not arbitrary; it’s dictated by several factors:
- Valence electrons: The number of valence electrons directly influences the number of electrons a metal atom will lose to achieve a stable configuration.
- Electrostatic forces: The attractive forces between the positively charged nucleus and the negatively charged electrons determine the energy needed to remove electrons.
- Electron shielding: Inner electrons shield the outer electrons from the full positive charge of the nucleus, influencing the ease of electron removal.
- Size of the atom: Larger atoms generally have lower ionization energies, meaning they more readily lose electrons.
Rare Exceptions: When Metals Might Seem to Form Anions
While overwhelmingly metals form cations, there are extremely rare instances where they might appear to form anions. These are highly specialized cases and don't represent the typical behavior of metals:
-
Zintl phases: These are intermetallic compounds containing alkali or alkaline earth metals and post-transition metals. In some Zintl phases, the electronegativity difference between the elements is small enough that the alkali or alkaline earth metals might formally be assigned negative oxidation states. However, it is crucial to understand that this is a formal assignment based on electron counting and does not imply the formation of true metal anions in the same way as non-metal anions. The bonding in these phases is complex and involves significant electron delocalization.
-
High-pressure conditions: Under extreme pressure, the electronic structure and properties of materials can change dramatically. In some cases, this can lead to the formation of unusual compounds where metals formally exhibit negative oxidation states. These are highly specialized and often occur only under extreme conditions that are not encountered under normal circumstances.
It's important to stress that even in these exceptional cases, the behavior of metals is not truly analogous to the anion formation seen in non-metals. The underlying bonding mechanisms are significantly different.
The Importance of Cation Formation in Various Chemical Processes
The tendency of metals to form cations is crucial for understanding a wide range of chemical and physical processes.
Ionic Compounds and Crystal Structures
The formation of metal cations is fundamental to the formation of ionic compounds. The electrostatic attraction between metal cations and non-metal anions drives the formation of crystalline solids with specific, predictable structures. The arrangement of ions in these structures is determined by factors like the size and charge of the ions, and this, in turn, impacts the physical properties of the compound, such as its melting point, hardness, and solubility.
Oxidation-Reduction Reactions (Redox Reactions)
Cation formation is a central feature of redox reactions. In these reactions, electrons are transferred between chemical species. When a metal loses electrons to form a cation, it undergoes oxidation. The simultaneous reduction of another species (usually a non-metal) completes the redox process.
Electrochemistry
The behavior of metals as electron donors is essential in electrochemistry. Metal electrodes in electrochemical cells can undergo oxidation, releasing electrons into the external circuit. This electron flow drives electrical current, which is the basis of batteries, fuel cells, and many other electrochemical devices.
Metallurgy and Material Science
Understanding the formation of metal cations is crucial in metallurgy and material science. Alloy formation, corrosion processes, and the design of new materials all depend on the ability of metals to lose electrons and interact with other elements.
Conclusion: Metals and Their Predominant Cationic Nature
The overwhelming tendency of metals to form cations is a cornerstone of chemistry. This behavior is directly linked to their electronic structure, low electronegativity, and low ionization energies. While rare exceptions exist under extreme conditions or in specialized compounds like Zintl phases, the formation of cations remains a defining characteristic of metals and is crucial for understanding numerous chemical and physical phenomena, from ionic compound formation and redox reactions to electrochemistry and materials science. The fundamental understanding of why metals form cations is essential for advancements in diverse fields.
Latest Posts
Latest Posts
-
Which Of The Following Is True About Monopolistic Competition
Apr 01, 2025
-
Which Of The Following Is The Site Of Translation
Apr 01, 2025
-
What Is The Atomic Number Of An Atom Equal To
Apr 01, 2025
-
Parathyroid Hormone Does All Of The Following Except
Apr 01, 2025
-
Which Structure Is Not Part Of A Neuron
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Do Metals Form Anions Or Cations . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
