Do Covalent Bonds Have High Solubility
News Leon
Mar 30, 2025 · 6 min read
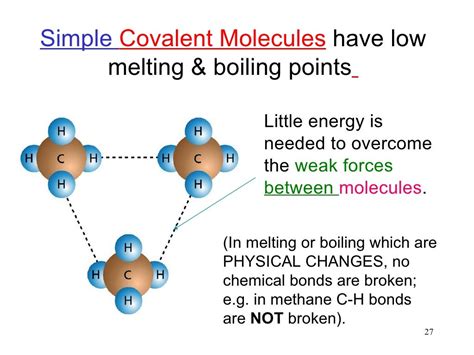
Table of Contents
Do Covalent Bonds Have High Solubility? A Deep Dive into Molecular Interactions and Solubility
The solubility of a substance is a complex phenomenon determined by the interplay of various intermolecular forces. While the presence of covalent bonds doesn't directly dictate solubility, the nature of the covalent bonds, and consequently the resulting molecular properties, significantly influence a molecule's solubility in different solvents. This article delves into the intricate relationship between covalent bonds and solubility, exploring the factors that determine whether a covalently bonded substance will readily dissolve or remain insoluble.
Understanding Covalent Bonds and Their Impact on Molecular Properties
Covalent bonds arise from the sharing of electrons between atoms. This sharing leads to the formation of molecules, with properties dictated by the types of atoms involved and their arrangement within the molecule. Crucially for solubility, these properties influence the types of intermolecular forces the molecule can participate in.
Polarity: A Key Determinant
One crucial molecular property arising from covalent bonding is polarity. Polarity refers to the uneven distribution of electron density within a molecule. This uneven distribution occurs when one atom in a covalent bond is more electronegative than the other, pulling the shared electrons closer to itself. This creates a dipole moment, with one end of the molecule carrying a partial negative charge (δ-) and the other end carrying a partial positive charge (δ+).
Examples of polar covalent bonds: The bond between oxygen and hydrogen in water (H₂O) is a classic example. Oxygen is significantly more electronegative than hydrogen, leading to a polar molecule with a significant dipole moment. Similarly, the bonds in ammonia (NH₃) are polar due to the higher electronegativity of nitrogen.
Nonpolar covalent bonds: In contrast, nonpolar covalent bonds occur between atoms with similar electronegativities. The shared electrons are distributed relatively evenly, resulting in a nonpolar molecule with little to no dipole moment.
Examples of nonpolar covalent bonds: The bonds in methane (CH₄) are essentially nonpolar, as carbon and hydrogen have similar electronegativities. Similarly, many hydrocarbons (molecules composed primarily of carbon and hydrogen) exhibit nonpolar characteristics.
Intermolecular Forces: The Glue that Holds It Together (and Apart)
The polarity of a molecule dictates the types of intermolecular forces it can engage in. These forces are crucial in determining solubility as they govern the interactions between solute molecules and solvent molecules. The main types of intermolecular forces include:
-
Hydrogen bonding: A particularly strong type of dipole-dipole interaction occurring between a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. Water's exceptional properties are largely due to its extensive hydrogen bonding network.
-
Dipole-dipole interactions: Attractive forces between polar molecules due to their dipole moments. These forces are weaker than hydrogen bonds but still significantly influence solubility.
-
London Dispersion Forces (LDFs): These are the weakest intermolecular forces, arising from temporary fluctuations in electron distribution around atoms. Even nonpolar molecules experience LDFs, albeit weaker ones. The strength of LDFs increases with the size and molecular weight of the molecule.
Solubility: The "Like Dissolves Like" Principle
The solubility of a substance is governed by the fundamental principle of "like dissolves like." This means that polar solvents tend to dissolve polar solutes, while nonpolar solvents tend to dissolve nonpolar solutes.
Polar Covalent Compounds and Solubility
Polar covalent compounds, possessing significant dipole moments and often engaging in hydrogen bonding, tend to be soluble in polar solvents like water. This is because the strong intermolecular forces (hydrogen bonds and dipole-dipole interactions) between the solute and solvent molecules overcome the forces holding the solute molecules together. The solute molecules become effectively surrounded and stabilized by solvent molecules, resulting in dissolution.
Examples: Sugars (which contain many hydroxyl groups, -OH, capable of hydrogen bonding) are highly soluble in water. Similarly, many alcohols and carboxylic acids are soluble in water due to their polar nature and ability to form hydrogen bonds.
Nonpolar Covalent Compounds and Solubility
Nonpolar covalent compounds, lacking significant dipole moments and relying primarily on weak London Dispersion Forces, generally exhibit low solubility in polar solvents. The weak interactions between nonpolar solute molecules and polar solvent molecules are insufficient to overcome the intermolecular forces within the solute, resulting in limited dissolution.
However, nonpolar covalent compounds tend to be soluble in nonpolar solvents. In this case, the weak LDFs between solute and solvent molecules are comparable in strength, allowing for dissolution.
Examples: Oils and fats, consisting primarily of long hydrocarbon chains, are nonpolar and therefore insoluble in water but soluble in organic solvents such as hexane or benzene.
Factors Influencing Solubility Beyond Polarity
While polarity plays a dominant role, other factors can influence the solubility of covalently bonded compounds:
-
Molecular size and shape: Larger molecules generally exhibit lower solubility due to the increased influence of LDFs, which can hinder interaction with the solvent. Molecular shape also plays a role, as a compact shape may enhance solubility compared to a more extended shape.
-
Presence of functional groups: The presence of specific functional groups within a molecule can significantly affect its solubility. For example, the presence of hydroxyl (-OH), carboxyl (-COOH), or amino (-NH₂) groups increases the polarity and hydrogen bonding capability, leading to enhanced solubility in polar solvents.
-
Temperature: Solubility often increases with temperature, as the increased kinetic energy facilitates the overcoming of intermolecular forces.
-
Pressure: Pressure has a more significant impact on the solubility of gases than liquids or solids. Increasing pressure generally increases the solubility of gases in liquids.
Case Studies: Illustrative Examples
Let's examine specific examples to reinforce our understanding:
1. Ethanol (CH₃CH₂OH): Ethanol is soluble in water. Despite having a nonpolar hydrocarbon chain, the polar hydroxyl (-OH) group allows for strong hydrogen bonding with water molecules, leading to significant solubility.
2. Hexane (CH₃(CH₂)₄CH₃): Hexane is a nonpolar hydrocarbon. It is insoluble in water but readily dissolves in nonpolar solvents like benzene. The weak LDFs between hexane molecules are comparable to those between hexane and benzene, leading to solubility.
3. Glucose (C₆H₁₂O₆): Glucose is a polar molecule with multiple hydroxyl (-OH) groups. These groups enable strong hydrogen bonding with water molecules, resulting in high solubility in water.
4. Benzene (C₆H₆): Benzene is a nonpolar aromatic hydrocarbon with delocalized electrons. It is insoluble in water due to its nonpolar nature but soluble in nonpolar solvents.
Conclusion: A nuanced perspective on covalent bond solubility
The solubility of covalently bonded compounds is not solely determined by the presence of covalent bonds. Instead, the crucial factor is the overall polarity of the molecule, which dictates the strength of its intermolecular interactions with potential solvents. The principle of "like dissolves like" elegantly summarizes this relationship: polar molecules dissolve in polar solvents, and nonpolar molecules dissolve in nonpolar solvents. However, other factors such as molecular size, shape, presence of functional groups, temperature, and pressure also play significant roles in determining the ultimate solubility of a covalently bonded substance. Understanding these factors is essential for predicting and explaining the solubility behaviour of diverse covalent compounds.
Latest Posts
Latest Posts
-
The Probability Of An Impossible Event Is
Apr 01, 2025
-
In What Way Are Energy And Nutrients Similar
Apr 01, 2025
-
Example Of Apology Letter For Lost Documents
Apr 01, 2025
-
A Relationship In Which Two Or More Species Benefit
Apr 01, 2025
-
Which Of The Following Is Not A Physiological Need
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Do Covalent Bonds Have High Solubility . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
