Difference Between Specific Gravity And Density
News Leon
Mar 30, 2025 · 5 min read
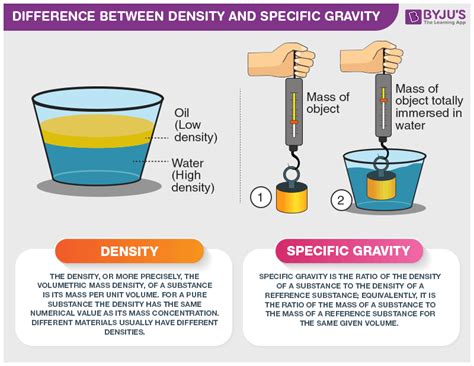
Table of Contents
Delving Deep: The Difference Between Specific Gravity and Density
Understanding the nuances between specific gravity and density is crucial in various scientific and engineering fields. While often used interchangeably in casual conversation, these two terms represent distinct concepts with different applications and calculations. This comprehensive guide will illuminate the differences, explore their applications, and offer practical examples to solidify your understanding.
Density: Mass per Unit Volume
Density, a fundamental property of matter, quantifies the amount of mass present in a given volume. It essentially tells us how tightly packed the atoms or molecules are within a substance. A higher density signifies more mass crammed into the same volume. The formula for density is straightforward:
Density = Mass / Volume
The standard unit for density is kilograms per cubic meter (kg/m³), although other units like grams per cubic centimeter (g/cm³) are also frequently used. Density is an intrinsic property, meaning it doesn't depend on the amount of substance present. A kilogram of gold will have the same density as a gram of gold. This intrinsic nature makes it a valuable identifier for materials.
Factors Affecting Density
Several factors can influence the density of a substance:
-
Temperature: As temperature increases, most substances expand, leading to a decrease in density. This is because the mass remains constant while the volume increases. Water is a notable exception, exhibiting unusual density behavior near its freezing point.
-
Pressure: Increasing pressure generally compresses a substance, reducing its volume and consequently increasing its density. This effect is particularly significant for gases.
-
Composition: The type and arrangement of atoms or molecules within a substance directly affect its density. Denser materials like gold have heavier atoms packed closely together, while less dense materials like wood have a more open structure with lighter atoms.
Specific Gravity: A Relative Measurement
Specific gravity, also known as relative density, is a dimensionless quantity that compares the density of a substance to the density of a reference substance, typically water at 4°C (39.2°F). It essentially tells us how much denser or less dense a substance is compared to water. The formula is:
Specific Gravity = Density of Substance / Density of Water (at 4°C)
Because it's a ratio of two densities, specific gravity is a unitless number. A specific gravity of 1 indicates that the substance has the same density as water. A specific gravity greater than 1 means the substance is denser than water, while a specific gravity less than 1 indicates that it is less dense than water.
Why Use Specific Gravity?
While density provides an absolute measure of mass per unit volume, specific gravity offers several advantages:
-
Simplicity: Specific gravity provides a readily understandable comparison. Knowing that a substance has a specific gravity of 2.5 instantly tells us it's 2.5 times denser than water.
-
Ease of Measurement: Measuring specific gravity is often easier than directly determining density, particularly using methods like hydrometry. Hydrometers are simple instruments that directly measure specific gravity.
-
Temperature Independence (within limits): As long as both the substance and the water are at the same temperature, fluctuations in temperature have less effect on the specific gravity value compared to the individual density values.
Key Differences Summarized
The table below highlights the key differences between density and specific gravity:
| Feature | Density | Specific Gravity |
|---|---|---|
| Definition | Mass per unit volume | Ratio of substance density to water density |
| Units | kg/m³, g/cm³, etc. | Unitless |
| Measurement | Direct measurement of mass and volume | Often measured using hydrometers or by comparing densities |
| Nature | Absolute | Relative |
| Application | Material identification, engineering design | Gemology, hydrology, industrial processes |
Applications of Density and Specific Gravity
Both density and specific gravity find widespread applications across various disciplines:
Density Applications:
-
Material Identification: Density is a crucial property used to identify unknown materials. Comparing the measured density of a sample to known densities of different materials helps determine its composition.
-
Engineering Design: Density plays a vital role in structural design, especially in aerospace and automotive engineering, where weight is a critical factor. Materials with high strength-to-weight ratios (high strength, low density) are preferred.
-
Fluid Mechanics: Density is fundamental in understanding fluid behavior, including buoyancy, pressure distribution, and fluid flow dynamics.
-
Geophysics: Density variations in the Earth's subsurface are used to infer geological structures and mineral deposits.
Specific Gravity Applications:
-
Gemology: Specific gravity is a key identifier in distinguishing between different gemstones, as they have unique specific gravity values.
-
Hydrology: Measuring the specific gravity of water samples helps determine the concentration of dissolved solids, providing insights into water quality and potential contamination.
-
Industrial Processes: In industries like brewing and food processing, specific gravity is used to monitor the concentration of solutions and to control various processes.
-
Medicine: Urine specific gravity is a common medical test indicating kidney function and hydration levels.
Practical Examples
Let's illustrate the concepts with a few examples:
Example 1: Suppose a sample of gold has a mass of 193.2 g and a volume of 10 cm³.
-
Density Calculation: Density = Mass / Volume = 193.2 g / 10 cm³ = 19.32 g/cm³
-
Specific Gravity Calculation: Assuming the density of water at 4°C is 1 g/cm³, the specific gravity of gold is 19.32 g/cm³ / 1 g/cm³ = 19.32
Example 2: A sample of a liquid has a specific gravity of 0.8. This means the liquid is less dense than water and would float on water.
Example 3: A hydrometer measures the specific gravity of a battery acid solution to be 1.285. This value indicates that the solution is 1.285 times denser than water, implying a high concentration of sulfuric acid.
Conclusion
While density and specific gravity are related concepts, they represent distinct measures of a substance's mass relative to its volume. Density provides an absolute value, while specific gravity offers a relative comparison to water. Understanding the nuances between these two concepts is essential for various scientific and engineering applications, from material identification and process control to geological surveys and medical diagnostics. By grasping these fundamental differences, you enhance your ability to interpret data and solve problems across a wide range of disciplines.
Latest Posts
Latest Posts
-
Genes On The Same Chromosome Are Said To Be
Apr 01, 2025
-
Which Of The Following Are Found In All Viruses
Apr 01, 2025
-
The Probability Of An Impossible Event Is
Apr 01, 2025
-
In What Way Are Energy And Nutrients Similar
Apr 01, 2025
-
Example Of Apology Letter For Lost Documents
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Specific Gravity And Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
