Diagram Of A Dry Cell Battery
News Leon
Apr 01, 2025 · 6 min read
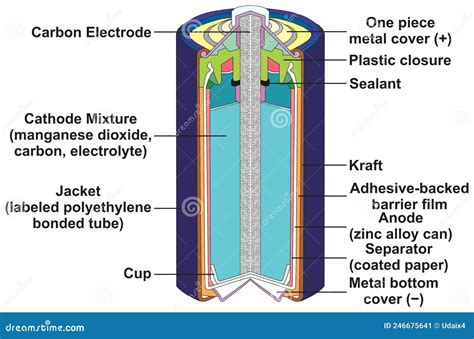
Table of Contents
A Deep Dive into the Diagram of a Dry Cell Battery: Construction, Chemistry, and Applications
Dry cell batteries are ubiquitous in our daily lives, powering everything from flashlights and toys to clocks and remote controls. Understanding their internal structure and the electrochemical processes within is crucial to appreciating their functionality and limitations. This article provides a comprehensive exploration of a dry cell battery diagram, delving into its components, the chemistry driving its operation, and its various applications. We will also address common misconceptions and explore future advancements in dry cell technology.
Anatomy of a Dry Cell Battery: A Detailed Diagram
A typical dry cell battery, specifically a zinc-carbon cell, comprises several key components, each playing a vital role in its operation. Let's dissect a representative diagram:
[Insert a high-quality, labelled diagram of a zinc-carbon dry cell battery here. The diagram should clearly show the following components:]
-
Zinc Container (Anode): This acts as the negative electrode (anode) and also serves as the battery's outer casing. The zinc is oxidized, releasing electrons and forming zinc ions (Zn<sup>2+</sup>). This is a crucial part of the electrochemical reaction.
-
Carbon Rod (Cathode): Positioned in the center, this is the positive electrode (cathode). It's an inert conductor, providing a pathway for electrons to flow back into the battery during discharge. It's surrounded by the cathode paste.
-
Electrolyte Paste: This paste fills the space between the zinc container and the carbon rod. It's a mixture of manganese dioxide (MnO<sub>2</sub>), ammonium chloride (NH<sub>4</sub>Cl), and carbon powder. The ammonium chloride is the electrolyte, facilitating the movement of ions between the electrodes. Manganese dioxide acts as the oxidizing agent, accepting electrons during the reaction. Carbon powder increases conductivity.
-
Separating Paper/Layer: A layer of absorbent paper or other porous material separates the electrolyte paste from the zinc container to prevent direct contact and control the rate of the reaction. This is sometimes omitted in simpler designs.
-
Insulating Layer (Optional): Many batteries include an additional layer of insulation surrounding the entire cell to prevent short circuits and enhance safety.
-
Metal Cap: This seals the top of the battery, providing structural integrity and electrical contact with the positive terminal.
The Electrochemical Reaction: Unpacking the Power Source
The function of a dry cell battery relies on a spontaneous redox reaction (reduction-oxidation). The zinc anode is oxidized, losing electrons, while the manganese dioxide cathode is reduced, gaining electrons. This electron flow constitutes the electric current.
Here's a simplified representation of the half-reactions:
Anode (Oxidation): Zn(s) → Zn<sup>2+</sup>(aq) + 2e<sup>-</sup>
Cathode (Reduction): 2MnO<sub>2</sub>(s) + 2NH<sub>4</sub><sup>+</sup>(aq) + 2e<sup>-</sup> → Mn<sub>2</sub>O<sub>3</sub>(s) + 2NH<sub>3</sub>(aq) + H<sub>2</sub>O(l)
Overall Reaction: Zn(s) + 2MnO<sub>2</sub>(s) + 2NH<sub>4</sub><sup>+</sup>(aq) → Zn<sup>2+</sup>(aq) + Mn<sub>2</sub>O<sub>3</sub>(s) + 2NH<sub>3</sub>(aq) + H<sub>2</sub>O(l)
This reaction generates a voltage of approximately 1.5 volts. The ammonium ions (NH<sub>4</sub><sup>+</sup>) in the electrolyte facilitate the movement of charge, ensuring the reaction continues. The build-up of zinc ions (Zn<sup>2+</sup>) and the formation of manganese(III) oxide (Mn<sub>2</sub>O<sub>3</sub>) contribute to the eventual depletion of the battery.
Different Types of Dry Cell Batteries: Variations on a Theme
While the zinc-carbon battery is the most basic type, several other variations exist, each with slightly different compositions and characteristics:
-
Alkaline Batteries: These offer a higher energy density and longer lifespan compared to zinc-carbon batteries. They use a potassium hydroxide (KOH) electrolyte instead of ammonium chloride and a zinc powder anode instead of a zinc container. The cathode often employs a more efficient manganese dioxide formulation.
-
Zinc-Chloride Batteries: These are a type of heavy-duty dry cell offering a compromise between performance and cost. They utilize a zinc chloride electrolyte.
-
Lithium Batteries (Primary): Though technically not strictly "dry cells" in the same way as zinc-carbon cells, primary lithium batteries share some similarities in their construction and non-rechargeable nature. They offer very high energy density and a long shelf life but can be more expensive.
Applications of Dry Cell Batteries: A Wide Range of Uses
Dry cell batteries find applications in numerous devices and situations due to their convenience, low cost, and wide availability. Some key applications include:
-
Portable Electronics: Flashlights, remote controls, toys, clocks, and portable radios are commonly powered by dry cell batteries.
-
Household Appliances: Some smaller household appliances, such as smoke detectors and clocks, utilize these batteries.
-
Industrial Applications: Certain sensors and monitoring devices in industrial settings employ dry cell batteries for their self-contained power source.
-
Medical Devices: Some medical devices, such as blood pressure monitors, utilize dry cells for their portable nature.
Environmental Concerns and Recycling: A Responsible Approach
Dry cell batteries contain materials that can be harmful to the environment if improperly disposed of. The heavy metals and chemicals in the electrolyte can leach into the soil and groundwater, causing pollution. Therefore, proper recycling is crucial:
-
Recycling Programs: Many communities have recycling programs specifically designed for batteries. Check with your local waste management authorities for details on drop-off locations and procedures.
-
Responsible Disposal: If recycling isn't readily available, ensure batteries are disposed of in a manner that minimizes environmental impact, following local guidelines.
Future Trends and Advancements in Dry Cell Technology
Research and development continue to refine dry cell battery technology, focusing on improvements in:
-
Energy Density: Researchers are striving to increase the amount of energy stored per unit volume or mass, leading to longer-lasting batteries.
-
Shelf Life: Extending the shelf life of batteries is a continuous goal, ensuring they remain functional for extended periods even when unused.
-
Sustainability: The use of more environmentally friendly materials and manufacturing processes is crucial for reducing the environmental impact of battery production and disposal.
-
Improved Safety: Enhancements in battery design and construction aim to minimize the risk of leaks, explosions, and other safety hazards.
Conclusion: A Versatile and Essential Power Source
Dry cell batteries remain an essential power source for countless applications. Understanding their internal structure, the underlying electrochemical reactions, and their limitations is critical for optimizing their use and managing their environmental impact. As technology progresses, we can expect further advancements in dry cell batteries, leading to more efficient, sustainable, and safer power sources for the future. By responsibly utilizing and recycling these essential components of our modern lives, we can contribute to a cleaner and more sustainable world.
Latest Posts
Latest Posts
-
Why Are There More Herbivores Than Carnivores
Apr 02, 2025
-
Domain And Range Y 1 X
Apr 02, 2025
-
What Is The Best Topic For Speech
Apr 02, 2025
-
Why Is The Vacuole Larger In Plant Cells
Apr 02, 2025
-
How To Initialize A Tuple In Python
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Diagram Of A Dry Cell Battery . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
