Determine The Number Of Possible Stereoisomers For The Compound Below.
News Leon
Mar 30, 2025 · 5 min read
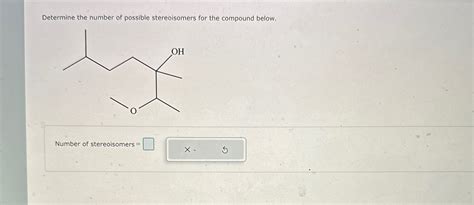
Table of Contents
Determining the Number of Possible Stereoisomers: A Comprehensive Guide
Determining the number of possible stereoisomers for a given organic compound is a fundamental skill in organic chemistry. Stereoisomers are molecules that have the same molecular formula and connectivity but differ in the three-dimensional arrangement of their atoms. Understanding how to predict the number of stereoisomers is crucial for comprehending the properties and behavior of molecules, particularly in areas like drug design and materials science. This article will provide a comprehensive guide to determining the number of possible stereoisomers, focusing on various types of stereoisomerism.
Understanding Stereoisomerism
Before diving into the calculation of stereoisomers, let's briefly review the different types:
1. Enantiomers:
Enantiomers are stereoisomers that are non-superimposable mirror images of each other. They possess chiral centers, which are carbon atoms bonded to four different groups. A molecule with n chiral centers can have a maximum of 2<sup>n</sup> stereoisomers. However, this only applies if the molecule lacks any internal planes of symmetry or meso compounds (discussed below).
2. Diastereomers:
Diastereomers are stereoisomers that are not mirror images of each other. They differ in the configuration at one or more chiral centers. Unlike enantiomers, diastereomers have different physical and chemical properties.
3. Meso Compounds:
Meso compounds are molecules that possess chiral centers but are achiral overall due to an internal plane of symmetry. They are superimposable on their mirror images, meaning they only exist as one stereoisomer, despite having chiral centers. The presence of a meso compound reduces the total number of stereoisomers compared to the 2<sup>n</sup> prediction.
4. Geometric Isomers (Cis-Trans Isomerism):
Geometric isomers, also known as cis-trans isomers or E/Z isomers, arise from restricted rotation around a double bond or a ring. Cis isomers have substituents on the same side of the double bond or ring, while trans isomers have them on opposite sides. Each geometric isomer can further exist as enantiomers if chiral centers are present in the molecule.
Calculating the Number of Stereoisomers: A Step-by-Step Approach
The process of determining the number of possible stereoisomers involves systematically identifying chiral centers, considering meso compounds, and accounting for geometric isomerism. Here's a structured approach:
-
Identify Chiral Centers: Carefully examine the molecule's structure and identify any carbon atoms bonded to four different groups. Each chiral center contributes to the potential number of stereoisomers.
-
Apply the 2<sup>n</sup> Rule (with Cautions): If the molecule possesses n chiral centers and no internal plane of symmetry or meso compounds, the maximum number of stereoisomers is 2<sup>n</sup>. This represents the potential for each chiral center to exist in either the R or S configuration.
-
Check for Meso Compounds: A meso compound will reduce the overall number of stereoisomers. If a molecule has an internal plane of symmetry, it's a meso compound and will only exist as one stereoisomer, regardless of the number of chiral centers. Carefully examine the molecule for such symmetry. This often involves mentally rotating or flipping portions of the molecule.
-
Account for Geometric Isomerism: If double bonds or rings are present, consider the possibilities of cis and trans (or E/Z) isomerism. Each geometric isomer will potentially exist as multiple stereoisomers if chiral centers are also present. Multiply the number of stereoisomers from chiral centers by the number of geometric isomers.
-
Combine all possibilities: Once you’ve identified all potential sources of stereoisomerism, combine all the possibilities to calculate the total number of possible stereoisomers.
Illustrative Examples:
Let's apply this step-by-step approach to a few examples. For clarity, we will focus on compounds with a relatively low number of chiral centers.
Example 1: A Simple Molecule with Two Chiral Centers
Consider a molecule with two chiral centers. Let's assume, for the sake of this example, that no meso compound exists, and no geometric isomerism is present. Using the 2<sup>n</sup> rule, where n = 2, we predict a maximum of 2² = 4 stereoisomers. These would consist of two pairs of enantiomers.
Example 2: A Molecule with a Meso Compound
Imagine a molecule with two chiral centers, but this time, it possesses an internal plane of symmetry. In this scenario, despite having two chiral centers, the molecule is a meso compound. Consequently, it only has one stereoisomer. The 2<sup>n</sup> rule does not apply directly because the presence of the internal plane of symmetry eliminates the other possible stereoisomers.
Example 3: A Molecule with Both Chiral Centers and Geometric Isomerism
Consider a molecule with one chiral center and one double bond. The chiral center will give rise to two enantiomers (2<sup>1</sup> = 2). The double bond introduces cis-trans isomerism, resulting in two geometric isomers (cis and trans). Therefore, the total number of stereoisomers would be 2 (enantiomers) * 2 (geometric isomers) = 4 stereoisomers.
Example 4: A More Complex Scenario
Let's consider a more complex example, a molecule with three chiral centers and one double bond. Assuming no meso compounds exist, the three chiral centers could potentially lead to 2³ = 8 stereoisomers. However, the double bond introduces another two possibilities (cis and trans). Therefore, the total number of stereoisomers would be 8 (chiral centers) * 2 (geometric isomerism) = 16 stereoisomers.
Advanced Considerations:
In more complex molecules, additional factors might influence the number of stereoisomers:
- Conformational Isomers: These isomers differ in the rotation around single bonds. While often considered less significant than configurational isomers (enantiomers and diastereomers), conformational analysis can be important in certain situations.
- Atropisomers: These are stereoisomers arising from hindered rotation around a single bond, often due to steric hindrance. They are not easily interconverted at room temperature.
Conclusion:
Determining the number of possible stereoisomers for a compound requires a systematic approach that carefully considers all potential sources of stereoisomerism, including chiral centers, meso compounds, and geometric isomerism. While the 2<sup>n</sup> rule provides a starting point, it's crucial to account for any exceptions and to perform a thorough analysis of the molecule's symmetry. Understanding these principles is essential for anyone working with organic molecules, contributing significantly to fields ranging from pharmaceutical development to material science. With practice and careful consideration, you'll be able to accurately predict the number of stereoisomers for even the most complex molecules. Remember to always carefully examine the molecular structure and apply the rules systematically. This comprehensive approach ensures accuracy and strengthens your understanding of this fundamental aspect of organic chemistry.
Latest Posts
Latest Posts
-
The Ultimate Source Of All New Alleles Is
Apr 01, 2025
-
Distance Between A Line And A Plane
Apr 01, 2025
-
The Long Run Perfectly Competitive Equilibrium
Apr 01, 2025
-
How Are Hydrogen Bonds Different From Covalent
Apr 01, 2025
-
Find The Modulus Of The Following 1 Es002 1 Jpg
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Determine The Number Of Possible Stereoisomers For The Compound Below. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
