How Are Hydrogen Bonds Different From Covalent
News Leon
Apr 01, 2025 · 6 min read
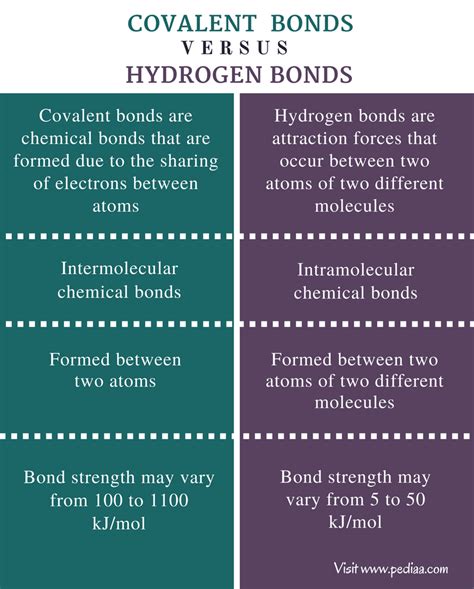
Table of Contents
How Are Hydrogen Bonds Different From Covalent Bonds? A Deep Dive into Molecular Interactions
Understanding the nuances of chemical bonding is crucial for comprehending the behavior of molecules and, ultimately, the properties of matter. While covalent bonds represent a strong, fundamental interaction within molecules, hydrogen bonds are a weaker, yet incredibly significant, type of intermolecular force. This article delves into the key differences between these two types of bonds, exploring their formation, strength, properties, and the crucial roles they play in various biological and chemical systems.
The Nature of Covalent Bonds: Sharing is Caring
Covalent bonds arise from the shared electrons between two atoms. This sharing occurs when atoms, particularly nonmetals, achieve a more stable electron configuration by pooling their valence electrons. The resulting shared electron pair creates a strong electrostatic attraction, holding the atoms together to form a molecule. The strength of a covalent bond is determined by several factors, including the electronegativity difference between the atoms involved and the number of shared electron pairs (single, double, or triple bonds).
Key Characteristics of Covalent Bonds:
- Strong Bond Strength: Covalent bonds are relatively strong, requiring significant energy to break. This strength contributes to the stability of molecules.
- Directional Bonds: The shared electron pair occupies a specific region in space, resulting in a directional bond. This directionality influences the molecular geometry and properties.
- Intramolecular Forces: Covalent bonds are intramolecular, meaning they occur within a molecule, holding atoms together to form the molecule itself.
- Electron Sharing: The defining characteristic is the sharing of valence electrons between atoms.
- Examples: Water (H₂O), methane (CH₄), glucose (C₆H₁₂O₆), and countless other organic and inorganic molecules are held together by covalent bonds.
Hydrogen Bonds: A Special Type of Intermolecular Force
Unlike covalent bonds, hydrogen bonds are intermolecular forces. This means they exist between molecules rather than within them. Specifically, they are a type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule (or even a different part of the same large molecule).
The Formation of a Hydrogen Bond:
The highly electronegative atom (e.g., oxygen in water) attracts the shared electrons in the covalent bond with hydrogen much more strongly. This creates a partial positive charge (δ+) on the hydrogen atom and a partial negative charge (δ-) on the electronegative atom. This creates a dipole moment. The partially positive hydrogen atom is then attracted to the partially negative atom of a neighboring molecule, forming a hydrogen bond.
Key Characteristics of Hydrogen Bonds:
- Weaker than Covalent Bonds: Hydrogen bonds are significantly weaker than covalent bonds. This means they are easier to break.
- Non-directional (relatively): While there is some directionality to the bond, it's less rigid than covalent bonds. The hydrogen bond is strongest when the hydrogen atom is aligned directly with the electronegative atom it is interacting with, but deviations from this optimal geometry still result in a bond.
- Intermolecular Forces: They are intermolecular, acting between molecules. They are crucial in determining the physical properties of substances, particularly those involving water.
- Electrostatic Attraction: The attraction is primarily electrostatic, arising from the interaction between partial charges.
- Examples: The cohesion and adhesion of water molecules, the structure of DNA and proteins (alpha-helices and beta-sheets), and the interactions between enzyme and substrate are all critically dependent on hydrogen bonds.
A Comparative Analysis: Covalent vs. Hydrogen Bonds
| Feature | Covalent Bond | Hydrogen Bond |
|---|---|---|
| Bond Type | Intramolecular | Intermolecular |
| Strength | Strong | Weak |
| Bond Formation | Sharing of electron pairs | Electrostatic attraction between dipoles |
| Atoms Involved | Typically nonmetals | Hydrogen bonded to a highly electronegative atom and another electronegative atom |
| Directionality | Highly directional | Relatively less directional |
| Energy Required to Break | High | Low |
| Effect on Properties | Determines molecular shape and reactivity | Influences physical properties like boiling point, melting point, and solubility |
The Significance of Hydrogen Bonds in Biological Systems
Hydrogen bonds are crucial for life as we know it. Their relatively weak nature allows for the formation and breakage of bonds, which is essential for dynamic biological processes.
Hydrogen Bonds in Water:
Water's unique properties, including its high boiling point, surface tension, and excellent solvent capabilities, are all a direct consequence of its extensive hydrogen bonding network. Each water molecule can form up to four hydrogen bonds with neighboring water molecules, creating a cohesive and stable structure.
Hydrogen Bonds in Proteins:
Hydrogen bonds play a crucial role in the secondary structure of proteins. Alpha-helices and beta-sheets, essential components of protein structure and function, are stabilized by hydrogen bonds between amino acid residues. These interactions ensure the protein folds into a specific three-dimensional shape, which is critical for its biological activity.
Hydrogen Bonds in Nucleic Acids (DNA and RNA):
The double helix structure of DNA is stabilized by hydrogen bonds between complementary base pairs (adenine with thymine, and guanine with cytosine). These bonds hold the two strands of the DNA molecule together, allowing for accurate replication and transcription of genetic information. Similar hydrogen bonding interactions are also important in RNA structure and function.
Hydrogen Bonds in Other Chemical Systems
Beyond biological systems, hydrogen bonds influence the properties of various chemical compounds. They contribute to the solubility of certain substances in water, the boiling points and melting points of many molecules, and even the formation of crystalline structures. For example, the relatively high boiling point of ammonia (NH₃) is attributed to the hydrogen bonds formed between ammonia molecules.
Differentiating Between Covalent and Hydrogen Bonds: A Practical Approach
The key to differentiating between these bond types lies in understanding their nature and the atoms involved. Covalent bonds involve the sharing of electrons between atoms within a molecule, leading to strong, relatively inflexible links. Hydrogen bonds are weaker intermolecular interactions involving a partially positive hydrogen atom and a partially negative electronegative atom.
To identify the bond type, ask yourself:
- Are the atoms directly bonded? If yes, it's likely a covalent bond. If the atoms are in separate molecules (or distant parts of a large molecule) interacting through the partially charged hydrogen, it's a hydrogen bond.
- Are the atoms involved highly electronegative? Hydrogen bonds invariably involve hydrogen bonded to a highly electronegative atom (O, N, or F).
- What is the strength of the interaction? Covalent bonds are much stronger and require significantly more energy to break.
Conclusion: The Interplay of Forces
While vastly different in strength and nature, both covalent and hydrogen bonds play indispensable roles in shaping the world around us. Covalent bonds form the backbone of molecules, defining their structure and reactivity. Hydrogen bonds, while weaker, are the orchestrators of intricate molecular interactions, dictating the properties of substances and driving essential biological processes. Understanding the differences and the subtle interplay between these forces is critical for comprehending the complexity of chemistry and biology. From the simplicity of a water molecule to the elegance of a protein's three-dimensional structure, the dance between covalent and hydrogen bonds is a fundamental choreography of the molecular world.
Latest Posts
Latest Posts
-
Distance Between Earth And Moon In Light Years
Apr 02, 2025
-
Which Of The Following Is Chemically Inert Unreactive
Apr 02, 2025
-
The Standard Unit For Measuring Volume Is
Apr 02, 2025
-
Materials Like Rubber That Resist The Flow Of E
Apr 02, 2025
-
What Are The Units Of Conductance
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Are Hydrogen Bonds Different From Covalent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
