Density Of Water At 4 Degrees Celsius
News Leon
Mar 23, 2025 · 5 min read
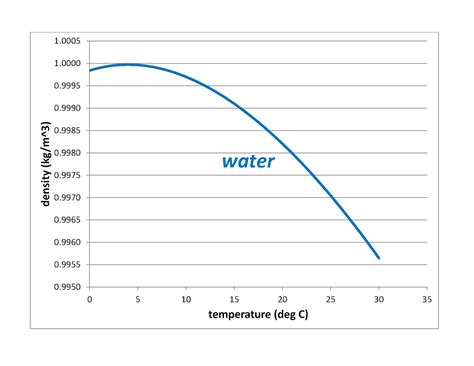
Table of Contents
The Unique Density of Water at 4 Degrees Celsius: A Deep Dive
Water, a seemingly simple molecule (H₂O), exhibits remarkably complex behavior, particularly regarding its density. Unlike most substances, water's density doesn't decrease linearly with decreasing temperature. Instead, it reaches its maximum density at a unique temperature: 4 degrees Celsius (39.2 degrees Fahrenheit). This seemingly insignificant detail has profound implications for aquatic life, weather patterns, and even the very existence of life on Earth as we know it. This article delves into the science behind this unique property, exploring its causes, consequences, and broader significance.
Understanding Density: Mass vs. Volume
Before we explore the anomaly of water's density at 4°C, let's establish a fundamental understanding of density itself. Density is a measure of how much mass is contained within a given volume. It's calculated as mass divided by volume (ρ = m/V). The units commonly used are grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). A higher density indicates that more mass is packed into a smaller volume.
Most substances, when cooled, contract, leading to an increase in density. This is because lower temperatures reduce the kinetic energy of molecules, causing them to move less and occupy less space. However, water defies this general rule.
The Hydrogen Bond: The Key to Water's Anomaly
The unusual density behavior of water stems from the unique nature of its intermolecular forces – specifically, hydrogen bonds. A hydrogen bond is a relatively weak electrostatic attraction between a hydrogen atom bonded to a highly electronegative atom (like oxygen in water) and another electronegative atom in a different molecule.
In liquid water, these hydrogen bonds constantly form and break. At higher temperatures, the molecules have greater kinetic energy, leading to more chaotic movement and weaker hydrogen bonds. As the temperature decreases, the molecules slow down, allowing more stable hydrogen bonds to form.
The Crystalline Structure of Ice: A Less Dense Arrangement
Below 4°C, the formation of hydrogen bonds becomes dominant. These bonds arrange the water molecules into a specific crystalline structure characteristic of ice. This structure is an open, hexagonal lattice, leaving significant empty space between the molecules. This open structure is why ice is less dense than liquid water, causing ice to float.
Above 4°C, the thermal energy is sufficient to disrupt the highly ordered structure of ice, allowing water molecules to move more freely. This results in a more compact arrangement, leading to an increase in density. However, the influence of hydrogen bonds continues to affect the density, resulting in a maximum density at 4°C.
The Density Maximum at 4°C: A Balancing Act
The density maximum at 4°C represents a delicate balance between two competing factors:
- Hydrogen bonding: The tendency of water molecules to form hydrogen bonds, promoting a more ordered, less dense structure.
- Thermal motion: The kinetic energy of water molecules, tending to disrupt the hydrogen bonds and lead to a more compact, denser structure.
At temperatures above 4°C, thermal motion is strong enough to overcome the influence of hydrogen bonding, leading to a decrease in density as temperature increases. Below 4°C, the formation of increasingly stable hydrogen bonds begins to dominate, leading to a decrease in density as the water approaches freezing. The point where these forces balance is precisely 4°C.
Implications of Water's Unique Density
The fact that water's density is maximum at 4°C has profound implications across various fields:
Aquatic Ecosystems:
- Winter survival of aquatic life: The fact that ice floats insulates the water below from extremely cold temperatures. This creates a layer of warmer water near the bottom of lakes and rivers, allowing aquatic organisms to survive through winter. Without this phenomenon, aquatic life would likely perish in most cold climates. This is a crucial factor in the sustenance of diverse aquatic ecosystems.
Global Climate Regulation:
- Ocean currents: The density differences in water at various temperatures drive ocean currents, which play a significant role in distributing heat around the globe. These currents moderate temperature differences between the equator and the poles, influencing global weather patterns. This impact on global climate regulation is enormous.
Chemistry and Biology:
- Solvent properties of water: Water's unique properties, including its high density, contribute to its exceptional ability to dissolve many substances, making it an ideal solvent for various biological processes. This characteristic is vital for life itself. The anomalous density of water is an essential factor in many chemical and biological reactions.
Research and Further Exploration
The unique density behavior of water has been a subject of extensive scientific research. Scientists continue to investigate the intricate details of hydrogen bonding and its influence on water's properties at various temperatures and pressures. This ongoing research aims to deepen our understanding of water's fundamental characteristics and their implications for various phenomena. Advanced computational techniques and experimental methods are constantly being developed to unravel the complexities of water's behavior.
Conclusion: A Remarkable Property with Profound Consequences
The maximum density of water at 4°C is not merely a curious scientific fact; it's a fundamental property with profound consequences for life on Earth. This seemingly small detail has enormous ramifications for aquatic ecosystems, global climate patterns, and the very chemistry of life itself. The interplay between hydrogen bonding and thermal motion, leading to this unique behavior, is a testament to the complexity and remarkable properties of this seemingly simple molecule. Further research continues to unveil the intricate details of this phenomenon, solidifying its position as a cornerstone of our understanding of the natural world. Understanding this density anomaly allows for deeper insights into various fields, including hydrology, climatology, and biology, enriching our understanding of the planet and its intricate systems. The remarkable properties of water underpin the very existence of life on earth and continue to inspire scientific exploration.
Latest Posts
Latest Posts
-
What Is Not A Subatomic Particle
Mar 25, 2025
-
All Populations Of All Species In A Given Area
Mar 25, 2025
-
Zn Hcl Zncl2 H2 Balance Equation
Mar 25, 2025
-
What Is The Unit Of Entropy
Mar 25, 2025
-
What Is 40 Percent Of 36
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Density Of Water At 4 Degrees Celsius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
