Density Of Water At 4 Degree Celsius
News Leon
Apr 01, 2025 · 5 min read
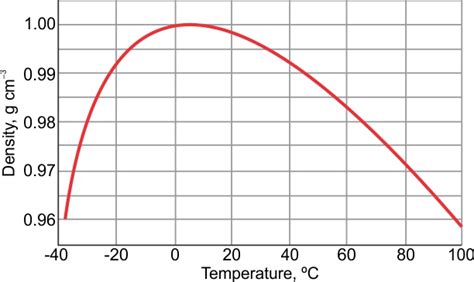
Table of Contents
The Unique Density of Water at 4 Degrees Celsius: A Deep Dive
Water, a seemingly simple molecule (H₂O), exhibits remarkably complex behavior, especially concerning its density. Unlike most substances, water doesn't exhibit a linear relationship between temperature and density. This anomaly has profound implications for life on Earth and numerous scientific applications. This article delves into the unique density of water at 4° Celsius, exploring its underlying causes, consequences, and significance.
Understanding Density: A Fundamental Concept
Density, simply put, is the mass of a substance per unit volume. It's typically expressed in grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). For most substances, density increases as temperature decreases because the molecules pack more closely together. Water, however, defies this general rule.
The Anomalous Behavior of Water: Why is it Unique?
The unusual density behavior of water stems from its molecular structure and the nature of hydrogen bonding. Water molecules are polar, meaning they possess a slightly positive end (hydrogen atoms) and a slightly negative end (oxygen atom). This polarity allows water molecules to form hydrogen bonds with each other – relatively strong intermolecular forces.
Hydrogen Bonding: The Key Player
Hydrogen bonds are responsible for many of water's exceptional properties, including its high boiling point, high surface tension, and its density anomaly. At temperatures above 4°C, the kinetic energy of water molecules overcomes the attractive forces of hydrogen bonds. Molecules move more freely, leading to a less dense structure.
The Crucial Role of Temperature
As the temperature of water decreases towards 4°C, the kinetic energy decreases, allowing hydrogen bonds to form more efficiently. This leads to a more ordered, open crystalline structure. However, below 4°C, the formation of ice crystals takes precedence. The formation of these crystals involves a significant expansion in volume, resulting in a decrease in density. This is why ice floats on water – a phenomenon crucial for aquatic life.
The Maximum Density Point: 4° Celsius
The density of water reaches its maximum at 4°C (39.2°F) at standard atmospheric pressure. At this temperature, the balance between the kinetic energy of the molecules and the strength of hydrogen bonding results in the most compact arrangement of water molecules. Any deviation from 4°C, either higher or lower, results in a less dense structure.
Density Variations Above and Below 4°C
- Above 4°C: As temperature increases, the increased kinetic energy disrupts the hydrogen bonds, causing the molecules to move further apart, resulting in decreased density.
- Below 4°C: The formation of ice crystals begins, leading to a significant expansion in volume and a consequent decrease in density. The crystalline structure of ice has a less efficient packing of molecules compared to the liquid state at 4°C.
The Significance of Water's Density Anomaly
The unique density behavior of water has profound implications for various aspects of the environment and life itself:
1. Aquatic Life and Ecosystem Stability
The fact that ice floats on water is vital for aquatic ecosystems. During winter, a layer of ice forms on the surface of lakes and rivers, insulating the water beneath. This prevents the entire body of water from freezing solid, allowing aquatic organisms to survive. Without this phenomenon, many aquatic ecosystems would be uninhabitable.
2. Ocean Currents and Global Climate Regulation
The density differences in water at various temperatures drive ocean currents. Cold, dense water sinks, while warmer, less dense water rises, creating a global circulation system that plays a critical role in regulating global climate. This density-driven circulation helps distribute heat around the planet, influencing weather patterns and temperature distributions.
3. Biological Processes
The density of water influences numerous biological processes. The buoyancy of water supports aquatic organisms, allowing them to move and feed efficiently. The unique properties of water also influence the solubility of substances, impacting biological reactions and nutrient transport.
4. Industrial Applications
Understanding the density of water is crucial in many industrial applications. Accurate measurements of density are vital in various industries, including chemical processing, food and beverage production, and environmental monitoring. The density of water changes with temperature and pressure. The maximum density at 4 °C is important for calibration and accuracy in these measurements.
Measuring the Density of Water
The density of water can be determined experimentally using various methods. One common method involves measuring the mass and volume of a known quantity of water using precise instruments such as an analytical balance and a volumetric flask. Density is then calculated by dividing the mass by the volume.
Factors Affecting Water Density Measurements
Several factors can affect the accuracy of water density measurements, including:
- Temperature: Precise temperature control is essential, as small variations in temperature significantly impact density.
- Pressure: Pressure also influences density, particularly at high pressures.
- Purity of Water: The presence of dissolved impurities can affect the density of water. Pure water is necessary for precise measurements.
- Calibration of Instruments: Accurate density measurements rely on properly calibrated instruments, including balances and volumetric glassware.
Further Research and Exploration
The study of water's unique properties, particularly its density anomaly, remains a vibrant area of scientific research. Scientists continue to explore the intricate details of hydrogen bonding and its influence on water's behavior under various conditions. Further research is crucial for enhancing our understanding of this fundamental substance and its role in various natural processes. This includes investigating the behavior of water under extreme temperatures and pressures, as well as exploring the implications of water's properties for technological advancements.
Conclusion: The Significance of a Simple Molecule
The density of water at 4°C, though seemingly a small detail, is a remarkable phenomenon with far-reaching consequences. This seemingly simple anomaly is fundamental to the stability of aquatic ecosystems, global climate regulation, and numerous biological processes. A deep understanding of water's unique properties is crucial for advancing scientific knowledge and tackling numerous challenges facing our planet. Further research into the complexities of water’s behavior promises to unveil even more about this remarkable substance, solidifying its importance as the foundation of life on Earth. The seemingly simple H₂O molecule continues to fascinate and challenge scientists, highlighting the complex world hidden within the simplicity of its structure.
Latest Posts
Latest Posts
-
A Clique Is A Group Of
Apr 02, 2025
-
Determine The Quantity Of Molecules In 2 00 Moles Of P4
Apr 02, 2025
-
Which Of The Following Compounds Have The Same Empirical Formula
Apr 02, 2025
-
An Instrument Used To Measure Atmospheric Pressure Is Called A
Apr 02, 2025
-
Name The Region That Attaches Two Sister Chromatids
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Density Of Water At 4 Degree Celsius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
