Density A Physical Or Chemical Property
News Leon
Mar 29, 2025 · 7 min read
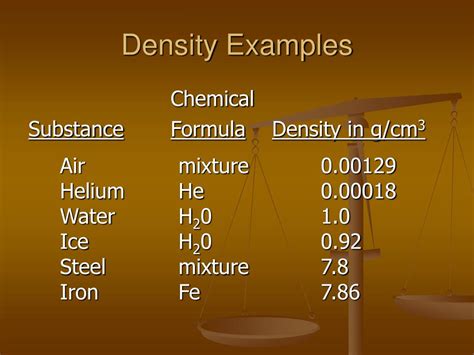
Table of Contents
- Density A Physical Or Chemical Property
- Table of Contents
- Density: A Physical Property – Understanding its Significance
- What is Density?
- Density as a Physical Property
- Factors Affecting Density
- 1. Temperature:
- 2. Pressure:
- 3. Composition:
- 4. Phase:
- Measuring Density
- Measuring the Mass:
- Measuring the Volume:
- Applications of Density
- 1. Materials Science:
- 2. Geology:
- 3. Oceanography:
- 4. Meteorology:
- 5. Medicine:
- 6. Food Science:
- 7. Environmental Science:
- Relative Density and Specific Gravity
- Density Anomalies: The Case of Water
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Density: A Physical Property – Understanding its Significance
Density, a fundamental concept in physics and chemistry, is a crucial property for characterizing materials and understanding their behavior. It's a measure of how much mass is packed into a given volume. While seemingly simple, understanding density unlocks insights into various scientific phenomena and has practical applications across numerous fields. This comprehensive guide will delve into the intricacies of density, exploring its definition, calculation, applications, and the factors that influence it.
What is Density?
Density (ρ, pronounced "rho") is defined as the mass per unit volume of a substance. In simpler terms, it describes how tightly packed the atoms or molecules are within a substance. A high-density material has a lot of mass crammed into a small volume, while a low-density material has the same mass spread over a larger volume. This is why a kilogram of lead feels much heavier than a kilogram of feathers – lead has a much higher density.
The formula for density is:
ρ = m/V
Where:
- ρ represents density (usually measured in kg/m³ or g/cm³)
- m represents mass (usually measured in kilograms or grams)
- V represents volume (usually measured in cubic meters or cubic centimeters)
This formula is straightforward and allows for the calculation of density given the mass and volume of a substance. However, the determination of mass and volume can vary depending on the state of matter (solid, liquid, or gas) and the techniques used for measurement.
Density as a Physical Property
Density is unequivocally classified as a physical property. This means it can be measured without changing the chemical composition of the substance. You can determine the density of a material without altering its chemical makeup. Contrast this with chemical properties, such as reactivity or flammability, which are observed only when a substance undergoes a chemical change. Measuring the density of water, for instance, doesn’t transform the water into something else. It remains water.
Factors Affecting Density
Several factors can influence the density of a substance. These include:
1. Temperature:
Temperature significantly impacts density, particularly in liquids and gases. As temperature increases, the kinetic energy of particles increases, causing them to move further apart. This expansion leads to a decrease in density. Conversely, a decrease in temperature results in a contraction and an increase in density. This is why hot air rises (it's less dense) and cold air sinks (it's more dense). The effect on solids is generally less pronounced than on liquids and gases.
2. Pressure:
Pressure primarily affects the density of gases. Increasing pressure forces gas molecules closer together, reducing the volume and increasing the density. This effect is less significant in liquids and solids due to the stronger intermolecular forces holding the particles together.
3. Composition:
The chemical composition of a substance directly influences its density. Different atoms and molecules have different masses and occupy different volumes, leading to variations in density. For example, the density of pure gold is significantly higher than the density of pure aluminum because gold atoms are much heavier and more closely packed. Alloys, mixtures of different metals, will have densities that are intermediate to those of the constituent metals depending on their proportions.
4. Phase:
The phase of a substance (solid, liquid, or gas) greatly affects its density. Generally, solids are the densest, followed by liquids, and then gases. This is due to the arrangement of particles in each phase. In solids, particles are closely packed and organized, resulting in high density. In liquids, particles are less ordered, leading to lower density. In gases, particles are widely dispersed, resulting in the lowest density. However, there are exceptions to this general rule (e.g., ice is less dense than liquid water).
Measuring Density
Measuring density requires determining both the mass and volume of a substance. Several methods can be used, depending on the state of matter and the desired accuracy:
Measuring the Mass:
Mass is typically measured using a balance. Electronic balances are commonly employed for their precision and ease of use.
Measuring the Volume:
The method for measuring volume depends on the state of matter:
-
Solids: For regularly shaped solids (cubes, spheres, cylinders), the volume can be calculated using geometric formulas. For irregularly shaped solids, water displacement is often used. The solid is submerged in a known volume of water, and the increase in water level is measured to determine the solid's volume.
-
Liquids: The volume of liquids is often measured using graduated cylinders, pipettes, or burettes, depending on the required accuracy.
-
Gases: Measuring the volume of gases is more complex and often involves using gas laws and specialized equipment.
Applications of Density
The concept of density has widespread applications in various fields:
1. Materials Science:
Density is a key factor in material selection for engineering applications. For example, the density of a material determines its weight, which is crucial in aerospace and automotive industries. Materials with high strength-to-weight ratios (high strength and low density) are highly desirable in these sectors.
2. Geology:
Density is used to identify minerals and rocks. Different minerals have characteristic densities, allowing geologists to distinguish them based on their mass and volume. This information is vital for geological mapping and resource exploration.
3. Oceanography:
Density variations in ocean water play a crucial role in ocean currents and marine life distribution. Density differences caused by temperature and salinity create density gradients that drive ocean circulation patterns.
4. Meteorology:
Density differences in air masses are responsible for weather patterns. Warm, less dense air rises, while cooler, denser air sinks, creating pressure gradients that drive winds and storms.
5. Medicine:
Density measurements are used in medical imaging techniques like bone density scans (DEXA scans) to assess bone health and diagnose osteoporosis.
6. Food Science:
Density is a critical parameter in food processing and quality control. It's used to determine the concentration of solids in liquids, such as in fruit juices or milk.
7. Environmental Science:
Density is used to monitor water pollution. Changes in water density can indicate the presence of pollutants or alterations in water quality.
Relative Density and Specific Gravity
Related to density are the concepts of relative density and specific gravity.
-
Relative Density: This refers to the ratio of the density of a substance to the density of a reference substance, usually water at 4°C. It's a dimensionless quantity.
-
Specific Gravity: This is essentially the same as relative density. It's the ratio of the density of a substance to the density of water at a specified temperature (usually 4°C). It's also a dimensionless quantity.
Density Anomalies: The Case of Water
Water exhibits a unique density anomaly. Most substances become denser as they solidify, but water becomes less dense when it freezes. This is due to the hydrogen bonding in water molecules. The hydrogen bonds create a crystal lattice structure in ice that is more open and less dense than the liquid state. This unusual property has significant implications for aquatic life, as ice floats on water, preventing bodies of water from freezing solid and allowing aquatic organisms to survive the winter.
Conclusion
Density, a fundamental physical property, plays a pivotal role in understanding the behavior of matter in various states and across different disciplines. Its straightforward calculation and far-reaching applications highlight its significance in scientific research, engineering, and numerous other fields. Understanding density allows us to explore the intricate connections between mass, volume, and the properties of materials, ultimately providing valuable insights into the world around us. From the movement of ocean currents to the design of aircraft, density is a concept that underpins many aspects of our understanding of the physical world. Further investigation into specific applications and variations in density across different materials and conditions will continue to yield valuable discoveries and enhance our knowledge of material science and its applications.
Latest Posts
Latest Posts
-
Clown Fish Living Among The Tentacles Of Sea Anemone
Apr 02, 2025
-
All Squares Are Rectangles But Not All Rectangles Are Squares
Apr 02, 2025
-
A Process By Which Information Is Exchanged Between Individuals
Apr 02, 2025
-
Select The Four Statements About Plasmodium That Are True
Apr 02, 2025
-
Greatest Common Factor Of 36 And 20
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Density A Physical Or Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
