Complete The Curved Arrow Mechanism Of The Following Double Elimination
News Leon
Apr 05, 2025 · 5 min read
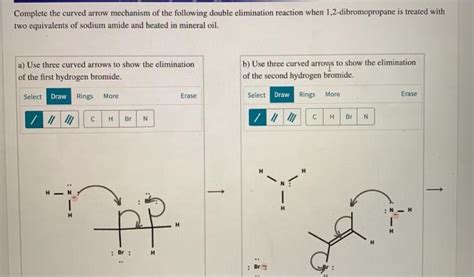
Table of Contents
Mastering the Curved Arrow Mechanism: A Deep Dive into Double Elimination Reactions
Organic chemistry, particularly reaction mechanisms, can feel like navigating a complex maze. Understanding curved arrow mechanisms is crucial for predicting reaction outcomes and designing synthetic routes. This article delves into the intricacies of double elimination reactions, focusing on completing curved arrow mechanisms and providing a comprehensive understanding of the underlying principles. We'll explore various examples, highlighting crucial steps and common pitfalls to avoid. By the end, you'll be confidently tackling even the most challenging double elimination problems.
What is a Double Elimination Reaction?
A double elimination reaction, also known as a dehydrohalogenation (if halogens are involved), involves the removal of two molecules (often small molecules like HX, H₂O, or other leaving groups) from a single reactant. This typically leads to the formation of a multiple bond (e.g., a double or triple bond) within the molecule. Unlike single elimination reactions, which usually proceed through a single step, double eliminations often involve a multi-step mechanism, making understanding the curved arrows crucial.
Understanding Curved Arrows: The Language of Reaction Mechanisms
Before we tackle double elimination mechanisms, let's review the fundamentals of curved arrows. These arrows represent the movement of electrons during a reaction, showing how bonds are broken and formed.
- Single-Barbed Arrow (Fishhook): Represents the movement of a single electron, typically found in radical reactions.
- Double-Barbed Arrow: Represents the movement of two electrons, crucial for depicting electron pair movement in most organic reactions.
Key Principles:
- Arrows always start from an electron-rich site: This is often a lone pair of electrons on an atom, a pi bond, or a negatively charged atom.
- Arrows always end at an electron-poor site: This is often a positively charged atom, or an atom with a partial positive charge.
- Always follow the octet rule (or expanded octet for elements in the third row and beyond): Ensure that all atoms involved in bond formation have a complete valence shell.
Common Types of Double Elimination Reactions
Several types of double elimination reactions exist, each with its own nuances and mechanistic pathways:
1. 1,2-Elimination: This involves the removal of two substituents from adjacent carbon atoms. This often leads to the formation of a double bond between those carbons. A classic example is the dehydrohalogenation of vicinal dihalides using a strong base.
2. 1,4-Elimination: This involves the removal of two substituents from carbon atoms separated by two other carbon atoms. This type of elimination often leads to the formation of a conjugated diene.
3. Elimination Reactions Involving Leaving Groups Other Than Halogens: Double elimination isn't limited to halogens. Other leaving groups, such as tosylate (-OTs), mesylate (-OMs), or even water molecules under specific conditions, can participate in double elimination processes.
Completing Curved Arrow Mechanisms: Step-by-Step Approach
Let's illustrate the process with a few examples. Remember, always focus on the movement of electron pairs:
Example 1: Dehydrohalogenation of a Vicinal Dihalide
Consider the dehydrohalogenation of 1,2-dibromoethane using a strong base like potassium tert-butoxide (t-BuOK). This reaction proceeds through two consecutive E2 elimination reactions:
Step 1: The strong base abstracts a proton from a carbon atom adjacent to the bromine. The electrons from the C-H bond form a new pi bond between the carbons, simultaneously breaking the C-Br bond.
(Curved Arrow Mechanism):
H Br
| |
CH₂ — CH₂ + t-BuO⁻ → CH₂=CHBr + t-BuOH + Br⁻
| |
Br H
Step 2: The same process repeats for the remaining bromo group. The strong base again abstracts a proton, forming another pi bond and eliminating the bromide ion.
(Curved Arrow Mechanism):
H Br
| |
CH₂=CHBr + t-BuO⁻ → CH≡CH + t-BuOH + Br⁻
| |
Br H
Important Considerations:
- Stereochemistry: E2 eliminations are stereospecific. The stereochemistry of the starting material influences the stereochemistry of the product. Anti-periplanar geometry is usually preferred for the leaving group and the proton being abstracted.
- Base Strength: Strong bases are required for these eliminations. Weak bases will generally not favor elimination pathways.
- Solvent Effects: The choice of solvent can influence the reaction rate and selectivity.
Example 2: Double Elimination Involving a Different Leaving Group
Let's consider a slightly more complex scenario, involving a molecule with tosylate (OTs) and a hydroxyl (-OH) group:
(Simplified Example, Requires more context for complete mechanism)
This reaction would likely proceed through a sequence of proton transfers and elimination steps. The exact mechanism would depend on the specific reaction conditions and the presence of any catalysts. The curved arrows would show the movement of electron pairs from the hydroxyl group to form a double bond, and subsequently the removal of the tosylate group. A detailed mechanism would involve several steps.
Example 3: 1,4-Elimination (A more challenging scenario)
1,4-Elimination reactions often lead to conjugated dienes. These mechanisms typically involve a series of proton transfers and elimination steps. Often, this type of elimination requires specific conditions or catalysts to facilitate the removal of the two groups from positions separated by two carbon atoms.
Advanced Considerations and Troubleshooting
- Competing Reactions: Double elimination reactions can compete with other reactions, such as substitution reactions (SN1 or SN2). The reaction conditions (temperature, solvent, base strength) are crucial in determining the preferred reaction pathway.
- Regioselectivity: In cases where multiple elimination pathways are possible, the reaction may show regioselectivity, favoring the formation of a specific product isomer.
- Stereoselectivity: As mentioned previously, the stereochemistry of the starting material significantly impacts the stereochemistry of the product. Anti-periplanar geometry is often crucial for successful E2 elimination.
Conclusion
Mastering curved arrow mechanisms is a cornerstone of organic chemistry. Double elimination reactions, while complex, can be systematically understood by meticulously tracking the movement of electrons. By carefully analyzing the starting material, reaction conditions, and the potential competing pathways, one can confidently predict the outcome of double elimination reactions and accurately depict their mechanisms using curved arrows. Practice is key – work through numerous examples to solidify your understanding and develop the intuition necessary to navigate these challenging reactions. Remember to always focus on the movement of electron pairs, respecting the octet rule (or expanded octet), and considering stereochemistry and other factors. With consistent effort, mastering double elimination mechanisms will become significantly easier, contributing to your overall success in organic chemistry.
Latest Posts
Latest Posts
-
2 Long Parallel Wires Carry Current
Apr 05, 2025
-
Examples Of Physical Characteristics In Geography
Apr 05, 2025
-
List One Way A Parallelogram And A Rhombus Are Different
Apr 05, 2025
-
Which Of The Following Is An Isoelectronic Series
Apr 05, 2025
-
The Reactions Of Glycolysis Occur In The
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Complete The Curved Arrow Mechanism Of The Following Double Elimination . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
