Chlorine Has Two Naturally Occurring Isotopes
News Leon
Apr 06, 2025 · 5 min read
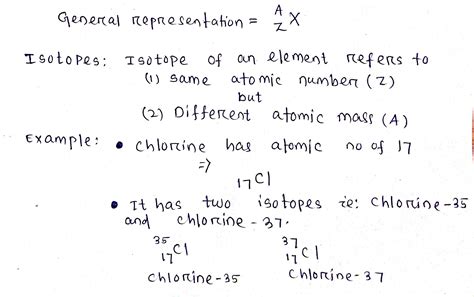
Table of Contents
Chlorine Has Two Naturally Occurring Isotopes: A Deep Dive into Isotopic Abundance and Applications
Chlorine, a ubiquitous element crucial to life and numerous industrial processes, is known for its unique isotopic composition. Unlike many elements found predominantly as a single isotope, chlorine boasts two naturally occurring isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl). Understanding the properties, abundance, and applications of these isotopes is key to appreciating chlorine's multifaceted role in the world around us. This in-depth exploration delves into the science behind chlorine isotopes, their implications in various fields, and the techniques used to study them.
Understanding Isotopes: A Quick Refresher
Before diving into the specifics of chlorine isotopes, let's briefly review the concept of isotopes. Isotopes are atoms of the same element that possess the same number of protons but differ in the number of neutrons. This difference in neutron count alters the atomic mass of the isotope, while leaving its chemical properties largely unchanged. The different isotopes of an element are typically denoted by the element's symbol followed by the mass number (the sum of protons and neutrons). For instance, ³⁵Cl indicates chlorine with 17 protons and 18 neutrons, whereas ³⁷Cl signifies chlorine with 17 protons and 20 neutrons.
The Two Faces of Chlorine: ³⁵Cl and ³⁷Cl
Chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl) are the two stable isotopes of chlorine. Their stability is a crucial factor in their widespread presence and applications. Unstable isotopes, or radioisotopes, undergo radioactive decay, transforming into different elements over time. The stability of ³⁵Cl and ³⁷Cl makes them ideal for various applications where long-term stability is essential.
Isotopic Abundance: A Matter of Proportion
The relative abundance of each isotope in nature is a significant characteristic. In the case of chlorine, ³⁵Cl significantly outnumbers ³⁷Cl. ³⁵Cl constitutes approximately 75.77% of naturally occurring chlorine, while ³⁷Cl accounts for the remaining 24.23%. This substantial difference in abundance has implications for various analytical techniques and applications.
Physical Properties: Subtle Differences
While the chemical properties of ³⁵Cl and ³⁷Cl are virtually identical, there are subtle differences in their physical properties. These differences, though minor, can be exploited in certain analytical techniques, such as mass spectrometry, which is capable of separating isotopes based on their mass-to-charge ratio. The difference in mass between the two isotopes influences their behavior in various physical processes.
Applications of Chlorine Isotopes
The unique isotopic composition of chlorine finds applications in diverse scientific fields. The consistent abundance ratio of ³⁵Cl and ³⁷Cl serves as a powerful tool for various analytical approaches.
1. Mass Spectrometry: Isotope Ratio Mass Spectrometry (IRMS)
Isotope Ratio Mass Spectrometry (IRMS) is a highly sensitive technique used to determine the isotopic ratios of elements in a sample. In the context of chlorine, IRMS can accurately measure the ³⁵Cl/³⁷Cl ratio. This ratio can reveal valuable information about various processes, such as:
- Environmental Studies: Investigating the sources and fate of pollutants, tracing water movement in hydrological cycles, and determining the origins of substances in environmental samples. Changes in the isotopic ratio can pinpoint specific sources of contamination.
- Forensic Science: Utilizing isotopic ratios as “fingerprints” to identify the source of materials in criminal investigations. The isotopic signature can help determine the origin of drugs or explosives, for instance.
- Geological Studies: Studying the formation and evolution of geological formations by examining the isotopic ratios in rocks and minerals. Changes in ratios can reveal past geological events.
2. Nuclear Magnetic Resonance (NMR) Spectroscopy
While less common for isotopic analysis compared to mass spectrometry, Nuclear Magnetic Resonance (NMR) spectroscopy can also provide information about chlorine isotopes. The different isotopes have distinct nuclear spins that influence their NMR spectra. This technique offers additional insight into the molecular environment of chlorine atoms.
3. Medical Applications: Radioisotopes of Chlorine
Although ³⁵Cl and ³⁷Cl are stable isotopes, some radioactive isotopes of chlorine exist. These radioisotopes, while not naturally occurring, are produced synthetically and find applications in nuclear medicine, though this application is relatively less common than other radioisotopes used in the field.
4. Industrial Processes: Monitoring and Control
Understanding the isotopic composition of chlorine is vital for monitoring and controlling industrial processes where chlorine is used. For example, in the production of chlorinated compounds, tracking the isotopic ratios can help optimize reaction conditions and product purity.
Factors Affecting Chlorine Isotope Ratios
The ³⁵Cl/³⁷Cl ratio is not constant across all samples. Various natural and anthropogenic processes can influence these ratios. These include:
- Fractionation: Differences in the physical and chemical behavior of ³⁵Cl and ³⁷Cl during various processes can lead to slight variations in their ratios. These variations are often small but detectable with high-precision techniques.
- Environmental Factors: Environmental conditions such as temperature, pressure, and the presence of other substances can affect isotope ratios.
- Anthropogenic Activities: Human activities, particularly industrial processes involving chlorine, can alter the isotopic composition of chlorine in the environment.
Challenges and Future Directions
While the study of chlorine isotopes has yielded significant insights, there are ongoing challenges and opportunities for future research. These include:
- Developing more accurate and precise analytical techniques: Continued refinement of IRMS and other isotopic analysis techniques is crucial for improving the accuracy and precision of measurements.
- Expanding the application of chlorine isotopes: Further investigation into the use of chlorine isotopes in various fields, such as environmental monitoring and forensic science, could unlock new possibilities.
- Understanding the impact of human activities: Research into the effects of anthropogenic activities on the isotopic composition of chlorine in the environment is vital for developing effective environmental management strategies.
Conclusion
The existence of two naturally occurring isotopes, ³⁵Cl and ³⁷Cl, adds a layer of complexity and richness to the study of chlorine. The differences in their abundance and subtle variations in their physical properties create opportunities for diverse applications, ranging from environmental monitoring to forensic investigations. By understanding the behavior and applications of these isotopes, scientists can gain valuable insights into various natural and anthropogenic processes. With ongoing advancements in analytical techniques and continued research, the role of chlorine isotopes in science and technology is poised to expand even further. The consistent presence and relatively easy measurement of these isotopes makes them powerful tools for exploring a multitude of scientific and practical problems. Their use promises to deepen our understanding of the world and refine techniques across many scientific fields.
Latest Posts
Latest Posts
-
Which Of The Following Is Equal To 1
Apr 07, 2025
-
What Is The Biggest Celestial Body In The Solar System
Apr 07, 2025
-
What Is The Lcm Of 4 5 And 6
Apr 07, 2025
-
H2so4 Ionic Or Molecular Acid Or Base
Apr 07, 2025
-
Evaluate 3 To The Power Of 4
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Chlorine Has Two Naturally Occurring Isotopes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
