Chemical Formula For Sodium And Oxygen
News Leon
Mar 28, 2025 · 5 min read
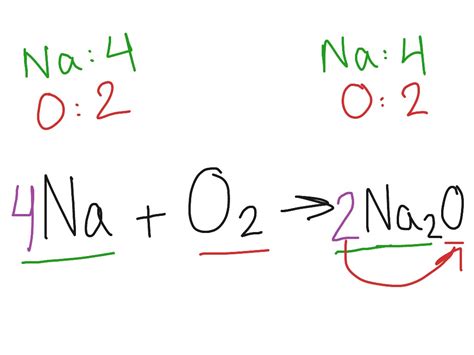
Table of Contents
The Chemical Formula for Sodium and Oxygen: Unveiling the Secrets of Sodium Oxide
Sodium and oxygen, two abundant elements found throughout nature, readily react to form a fascinating compound: sodium oxide. Understanding the chemical formula for this compound and the reactions that produce it is fundamental to comprehending various chemical processes and their applications. This article delves deep into the intricacies of sodium oxide, exploring its formation, properties, and significance in various fields.
Understanding Sodium and Oxygen: A Foundation for Understanding Sodium Oxide
Before diving into the specifics of sodium oxide, let's refresh our understanding of its constituent elements: sodium and oxygen.
Sodium (Na): The Alkali Metal
Sodium (Na), an alkali metal, is a highly reactive element characterized by its silvery-white appearance and softness. Its atomic number is 11, meaning it has 11 protons in its nucleus and 11 electrons orbiting the nucleus. Its single valence electron makes it extremely eager to participate in chemical reactions, readily losing this electron to achieve a stable electron configuration. This explains its high reactivity, especially with elements like oxygen.
Oxygen (O): The Life-Giving Element
Oxygen (O), a nonmetal, is crucial for life as we know it. It's a highly electronegative element, meaning it strongly attracts electrons. Its atomic number is 8, with 8 protons and 8 electrons. Oxygen readily accepts electrons to complete its outer electron shell, achieving a stable octet configuration. This tendency to gain electrons contributes to its powerful oxidizing properties.
The Chemical Formula: Na₂O – A Product of Ionic Bonding
When sodium and oxygen react, they engage in an ionic bond. This type of bond is formed through the electrostatic attraction between oppositely charged ions. Sodium, with its tendency to lose an electron, becomes a positively charged ion (Na⁺), a cation. Conversely, oxygen, with its tendency to gain electrons, becomes a negatively charged ion (O²⁻), an anion.
To achieve electrical neutrality, two sodium ions are needed for every one oxygen ion. This is because each oxygen ion requires two electrons to complete its octet, and each sodium ion provides only one. This leads to the chemical formula Na₂O, representing sodium oxide. This formula succinctly communicates the stoichiometric ratio of sodium and oxygen atoms within the compound.
The Reaction: A Vigorous Combustion
The reaction between sodium and oxygen is highly exothermic, meaning it releases a considerable amount of heat. This reaction typically manifests as a vigorous combustion when sodium metal is exposed to air. The reaction equation is as follows:
4Na(s) + O₂(g) → 2Na₂O(s)
This equation shows that four moles of solid sodium react with one mole of gaseous oxygen to produce two moles of solid sodium oxide. The reaction is characterized by a bright yellow flame and the production of significant heat. This is a classic example of a redox reaction, where sodium undergoes oxidation (loses electrons) and oxygen undergoes reduction (gains electrons).
Properties of Sodium Oxide: A Closer Look
Sodium oxide, Na₂O, is a white, crystalline solid at room temperature. It has several notable properties:
- High Melting Point: Na₂O possesses a relatively high melting point, reflecting the strong ionic bonds holding its crystal lattice together. Breaking these bonds requires a significant input of energy.
- Reactivity with Water: Sodium oxide is highly reactive with water, readily undergoing a reaction that produces sodium hydroxide (NaOH), a strong base:
Na₂O(s) + H₂O(l) → 2NaOH(aq)
This reaction is exothermic, releasing heat. The resulting sodium hydroxide solution is highly alkaline and corrosive.
- Reactivity with Acids: Sodium oxide is a basic oxide, meaning it readily reacts with acids. The reaction with acids like hydrochloric acid (HCl) leads to the formation of sodium chloride (NaCl) and water:
Na₂O(s) + 2HCl(aq) → 2NaCl(aq) + H₂O(l)
- Hygroscopic Nature: Sodium oxide is hygroscopic, meaning it readily absorbs moisture from the atmosphere. This absorption can lead to the formation of sodium hydroxide.
Applications of Sodium Oxide and Related Compounds: Exploring its Uses
While pure sodium oxide is not widely used in its pure form due to its high reactivity, its related compounds find extensive applications in various fields:
1. Industrial Applications:
-
Glass Manufacturing: Sodium oxide, often introduced as sodium carbonate (Na₂CO₃), is a crucial component in glass manufacturing. It acts as a flux, lowering the melting point of silica (SiO₂) and improving the workability of the glass melt.
-
Ceramic Industry: Similar to its role in glassmaking, sodium oxide is used in the ceramic industry to improve the properties of ceramic materials, influencing their melting point and viscosity.
-
Soap Production: Sodium hydroxide (NaOH), a product of the reaction between sodium oxide and water, is a fundamental component in the soap-making process through saponification.
2. Laboratory Applications:
-
Drying Agent: Although sodium oxide itself is highly reactive with water, its related compounds like sodium sulfate (Na₂SO₄) find use as drying agents in laboratories.
-
Chemical Reagent: Sodium hydroxide, derived from sodium oxide, serves as a vital chemical reagent in numerous laboratory procedures, including titrations and synthesis reactions.
3. Other Applications:
-
Sodium Lamps: Sodium vapor lamps utilize sodium in their operation, producing a characteristic yellow light.
-
Food Industry: Sodium compounds like sodium chloride (table salt) are ubiquitous in the food industry for flavoring and preservation.
Safety Precautions: Handling Sodium and Sodium Oxide
Both sodium metal and sodium oxide are highly reactive and require careful handling. Direct contact with skin can lead to burns and irritation. Inhaling sodium oxide dust can be harmful to the respiratory system. Therefore, appropriate safety measures, including the use of personal protective equipment (PPE) like gloves, safety glasses, and respirators, are essential when handling these substances. Work should always be conducted in well-ventilated areas to minimize the risk of exposure to hazardous fumes or dust.
Conclusion: A Comprehensive Overview of Sodium Oxide
This exploration of the chemical formula for sodium and oxygen, Na₂O, has unveiled the fascinating properties and diverse applications of sodium oxide and its related compounds. Understanding the nature of ionic bonding, the reaction mechanisms involved in its formation, and its reactivity is crucial to comprehending numerous chemical processes and industrial applications. Always remember that safety precautions are paramount when working with sodium and sodium oxide due to their inherent reactivity. Further research into specific applications and handling procedures should be conducted prior to any practical work involving these substances. The information presented here serves as a solid foundation for deeper exploration into the world of inorganic chemistry and the remarkable reactivity of sodium and oxygen.
Latest Posts
Latest Posts
-
What Is The Bond Order Of No
Mar 31, 2025
-
Two Small Metal Spheres Are Connected By A Wire
Mar 31, 2025
-
Which Of The Following Is An Endothermic Process
Mar 31, 2025
-
Cuanto Es 200 Cm En Metros
Mar 31, 2025
-
Every Natural Number Is An Integer True Or False
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Chemical Formula For Sodium And Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
