Charge Of Sodium Ion In Coulombs
News Leon
Mar 30, 2025 · 6 min read
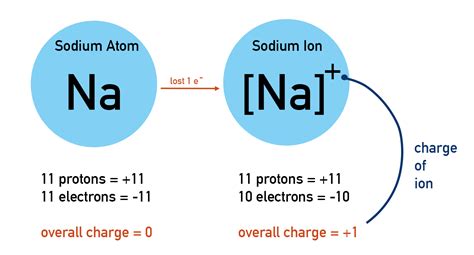
Table of Contents
Charge of a Sodium Ion in Coulombs: A Deep Dive into Electrostatics
The seemingly simple question, "What is the charge of a sodium ion in Coulombs?" opens a fascinating exploration into the world of electrostatics, atomic structure, and the fundamental units of charge. This article will delve deep into this topic, explaining not only the answer but also the underlying principles that govern it. We'll explore the concept of the elementary charge, the role of ionization in creating charged particles, and the practical implications of understanding ionic charge.
Understanding the Elementary Charge
Before we can determine the charge of a sodium ion, we must first grasp the concept of the elementary charge, often represented as 'e'. This is the fundamental unit of electric charge, the smallest unit of electric charge that can exist independently. Its value is approximately 1.602 x 10^-19 Coulombs. This means that every charged particle carries a charge that is an integer multiple of the elementary charge.
This discovery, a cornerstone of modern physics, revolutionized our understanding of matter and electricity. It established that charge is quantized—it exists in discrete packets, not as a continuous flow. This quantization is crucial in understanding how atoms and molecules interact, forming the basis of chemical bonding and numerous physical phenomena.
Sodium's Atomic Structure and Ionization
Sodium (Na), an alkali metal, occupies the third row of the periodic table. Its atomic number is 11, indicating that a neutral sodium atom possesses 11 protons in its nucleus and 11 electrons orbiting the nucleus. These electrons are arranged in specific energy levels or shells. The electron configuration is 2, 8, 1, signifying two electrons in the first shell, eight in the second, and one electron in the outermost or valence shell.
This single valence electron is relatively loosely bound to the atom. When a sodium atom interacts with other atoms or molecules, it readily loses this valence electron to achieve a more stable electronic configuration. This process is called ionization.
The Ionization Process and Charge Transfer
The ionization of a sodium atom involves the complete removal of its single valence electron. When this happens, the sodium atom is no longer electrically neutral. It loses a negative charge (the electron), leaving behind a net positive charge. This positively charged sodium atom is now called a sodium ion, represented as Na+.
The loss of a single electron means the sodium ion now has 11 protons and 10 electrons. The imbalance of one proton (positive charge) results in a net positive charge equal to the magnitude of the elementary charge.
Calculating the Charge of a Sodium Ion
Now we can calculate the charge of a sodium ion in Coulombs:
- Number of excess protons: 1
- Charge of one proton: +1.602 x 10^-19 Coulombs
- Total charge of the sodium ion: 1 * (+1.602 x 10^-19 Coulombs) = +1.602 x 10^-19 Coulombs
Therefore, the charge of a sodium ion (Na+) is +1.602 x 10^-19 Coulombs. This is equal in magnitude to the elementary charge, but positive instead of negative.
Significance of Ionic Charge in Chemical Reactions and Physical Phenomena
Understanding the charge of ions like Na+ is crucial in many areas of science and technology:
1. Chemical Bonding:
Ionic bonding, a fundamental type of chemical bond, arises from the electrostatic attraction between oppositely charged ions. The positive sodium ion readily forms ionic bonds with negatively charged ions, like chloride (Cl-), forming compounds like sodium chloride (NaCl), common table salt. The strong electrostatic forces between Na+ and Cl- hold the crystal lattice structure of NaCl together.
2. Electrochemistry:
Electrochemistry deals with the relationship between chemical reactions and electrical energy. Ionic charges play a critical role in electrochemical processes such as electrolysis, batteries, and fuel cells. The movement of ions across electrodes generates electric currents, powering numerous devices.
3. Biological Systems:
Sodium ions are essential for numerous biological processes. They are involved in nerve impulse transmission, muscle contraction, and fluid balance in living organisms. The selective permeability of cell membranes to ions like Na+ allows for the creation of electrical potentials across cell membranes, critical for cellular function.
4. Material Science:
Ionic compounds possess unique physical properties that are determined by their ionic charges and crystal structures. These properties find applications in various materials, including ceramics, glass, and semiconductors. The ionic conductivity of materials is crucial in applications like solid-state batteries and sensors.
Coulombs Law and Ionic Interactions
Coulomb's Law quantitatively describes the electrostatic force between charged particles. The law states that the force is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. For two point charges, q1 and q2, separated by a distance r, the force (F) is:
F = k * (q1 * q2) / r^2
where k is Coulomb's constant (approximately 8.987 x 10^9 N⋅m^2/C^2).
This law helps us understand the strength of interactions between ions. The magnitude of the charge plays a crucial role; larger charges result in stronger interactions. The distance also matters; closer ions interact more strongly.
In the case of sodium chloride, the strong electrostatic attraction between Na+ and Cl- ions due to their charges holds the crystal structure together. This interaction is responsible for the solid state of common salt at room temperature.
Beyond Sodium: Exploring Charges of Other Ions
The principles discussed for sodium ions apply to other ions as well. The charge of an ion depends on the number of electrons it has gained or lost compared to its neutral atomic state. For example:
- Magnesium ion (Mg2+): Loses two electrons, resulting in a charge of +3.204 x 10^-19 Coulombs.
- Chloride ion (Cl-): Gains one electron, resulting in a charge of -1.602 x 10^-19 Coulombs.
- Oxide ion (O2-): Gains two electrons, resulting in a charge of -3.204 x 10^-19 Coulombs.
The charge of an ion is always an integer multiple of the elementary charge, reflecting the discrete nature of electric charge.
Conclusion
The charge of a sodium ion, +1.602 x 10^-19 Coulombs, is a fundamental concept with wide-ranging implications. Understanding this charge, along with the principles of ionization and Coulomb's Law, provides a crucial foundation for comprehending various chemical reactions, physical phenomena, and biological processes. From the formation of ionic compounds to the functioning of biological systems, the charge of ions plays an essential role in shaping the world around us. This exploration has not only answered the initial question but also shed light on the fundamental nature of electric charge and its significance in the universe. Further exploration into these topics will reveal even greater depths of understanding of the physical world.
Latest Posts
Latest Posts
-
Which Element Has 2 Valence Electrons
Apr 01, 2025
-
Which Of The Following Statements Is True Of Proteins
Apr 01, 2025
-
Which Of The Following Statements About Receptor Mediated Endocytosis Is True
Apr 01, 2025
-
Above The Critical Temperature A Substance
Apr 01, 2025
-
Is Tarnish A Physical Or Chemical Change
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Charge Of Sodium Ion In Coulombs . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
