Cells Placed In A Hypotonic Solution Will
News Leon
Mar 29, 2025 · 6 min read
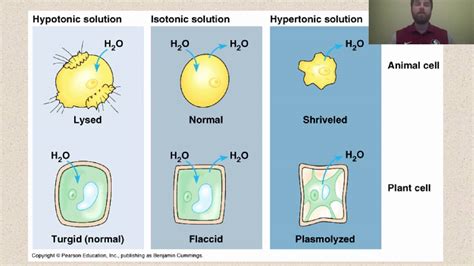
Table of Contents
Cells Placed in a Hypotonic Solution Will: A Deep Dive into Osmosis and Cell Behavior
Understanding how cells react to different environments is fundamental to biology. A key concept in this understanding is osmosis, the movement of water across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. When a cell is placed in a hypotonic solution, predictable changes occur due to this osmotic pressure. This article will delve into the detailed mechanisms of what happens when cells are placed in a hypotonic solution, exploring the variations in response depending on cell type and the implications for various biological processes.
What is a Hypotonic Solution?
Before exploring the effects on cells, let's define our terms. A hypotonic solution is one in which the concentration of solutes is lower outside the cell than inside the cell. This means the water concentration is higher outside the cell. This difference in solute concentration drives the movement of water. Remember, water moves to equalize the concentration of solutes on either side of the membrane.
Osmosis: The Driving Force
Osmosis is the passive movement of water molecules across a semipermeable membrane. This movement is driven by the water potential gradient, the difference in water potential between two areas. Water potential is influenced by both the concentration of solutes (solute potential) and the pressure exerted on the water (pressure potential). In a hypotonic solution, the water potential outside the cell is higher than inside the cell. This difference creates a driving force for water to move into the cell.
The Effects on Different Cell Types:
The effects of placing a cell in a hypotonic solution vary depending on whether the cell has a cell wall or not.
Animal Cells:
Animal cells lack a rigid cell wall. When an animal cell is placed in a hypotonic solution, water rushes into the cell via osmosis. The cell swells as it takes in water. If the influx of water is substantial and unchecked, the cell membrane can eventually burst, a process called lysis. This is because animal cell membranes are relatively fragile and lack the structural support to withstand significant pressure increases.
Examples:
- Red blood cells (erythrocytes) in distilled water: Red blood cells placed in pure water will swell and eventually lyse, releasing their hemoglobin into the surrounding solution. This is why intravenous fluids used in medicine are isotonic, matching the solute concentration of blood.
- Amoeba in freshwater: Amoebas are single-celled organisms that live in freshwater environments, which are generally hypotonic relative to their cytoplasm. They employ contractile vacuoles to pump excess water out of the cell, preventing lysis. This is a crucial adaptation for their survival.
Plant Cells:
Plant cells possess a rigid cell wall made primarily of cellulose. This cell wall provides structural support and prevents the cell from bursting even when placed in a hypotonic solution.
When a plant cell is placed in a hypotonic solution, water enters the cell via osmosis, causing the cell to swell. However, the cell wall prevents excessive swelling and bursting. Instead, the cell becomes turgid. The cell membrane pushes against the cell wall, creating turgor pressure. This turgor pressure is essential for maintaining the rigidity and shape of plant tissues. Turgor pressure is what keeps plants upright.
Examples:
- Plant cells in rainwater: Rainwater is generally hypotonic compared to the cytoplasm of plant cells. The cells become turgid, contributing to the overall firmness of the plant. Wilting occurs when plants lose turgor pressure due to water loss, often in response to a hypertonic environment.
- Guard cells and stomatal regulation: Guard cells, specialized cells surrounding stomata (pores in leaves), regulate gas exchange. Their turgor pressure, influenced by osmosis and solute concentration, controls the opening and closing of stomata, impacting photosynthesis and water loss.
Factors Influencing Osmotic Response:
Several factors can modulate the response of a cell to a hypotonic solution:
- Concentration Gradient: The steeper the concentration gradient (the larger the difference in solute concentration between the inside and outside of the cell), the faster the rate of water movement into the cell.
- Membrane Permeability: The permeability of the cell membrane to water influences the rate of osmosis. Aquaporins, specialized water channels, greatly increase membrane permeability to water.
- Cell Size and Shape: Larger cells with a greater surface area-to-volume ratio may experience a faster influx of water compared to smaller cells.
- Temperature: Temperature affects the rate of molecular movement, thereby influencing the rate of osmosis. Higher temperatures generally lead to faster osmosis.
- Solute Type: The type of solute present in the solution can also affect osmosis. Some solutes may be able to permeate the cell membrane, influencing the overall osmotic balance.
Biological Significance of Hypotonic Environments:
The ability of cells to respond appropriately to hypotonic environments is crucial for numerous biological processes:
- Water Uptake in Plants: The turgor pressure generated by osmosis in a hypotonic environment is vital for plant growth, nutrient uptake, and support.
- Maintaining Cell Volume: Organisms living in hypotonic environments have evolved mechanisms to regulate cell volume and prevent lysis. This is essential for maintaining cell function and integrity.
- Waste Removal: In some organisms, osmosis in a hypotonic environment can aid in the removal of metabolic waste products.
- Nutrient Absorption: Osmosis facilitates the movement of water and dissolved nutrients into cells.
Practical Applications and Research:
Understanding the effects of hypotonic solutions is paramount in several fields:
- Medicine: Intravenous fluid administration requires careful consideration of tonicity to prevent cell damage. Isotonic solutions are generally preferred to avoid cell lysis or crenation.
- Agriculture: Irrigation practices need to take into account soil tonicity to optimize water uptake by plant roots and prevent wilting.
- Food preservation: Osmotic pressure is used in food preservation techniques such as pickling and salting, which create hypertonic environments, drawing water out of microorganisms and inhibiting their growth.
- Cell Biology Research: Studying the effects of hypotonic solutions on cells provides valuable insight into membrane transport, cell signaling, and other cellular processes.
Conclusion:
The response of cells to hypotonic solutions is a fundamental aspect of cell biology. Whether a cell lyses, becomes turgid, or employs specialized mechanisms to regulate water uptake depends largely on its cell structure and the specific environmental conditions. This knowledge is critical not just for understanding basic biological principles but also for addressing practical applications in medicine, agriculture, and other fields. Further research continues to uncover the nuances of osmotic regulation and its significance in various biological systems, highlighting the ongoing importance of this seemingly simple yet profoundly impactful process. Understanding these cellular responses to hypotonic solutions continues to be a vital area of study with implications across various scientific and practical domains.
Latest Posts
Latest Posts
-
Which Of The Following Is An Example Of A Mixture
Mar 31, 2025
-
What Is The Reciprocal Of 1 6
Mar 31, 2025
-
What Mineral Is The Hardest Known Substance In Nature
Mar 31, 2025
-
Which Organelle Is Enclosed By A Double Membrane
Mar 31, 2025
-
Compare And Contrast An Ecosystem And A Habitat
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Cells Placed In A Hypotonic Solution Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
