Calculate The Molecular Mass Of Co2
News Leon
Mar 23, 2025 · 4 min read
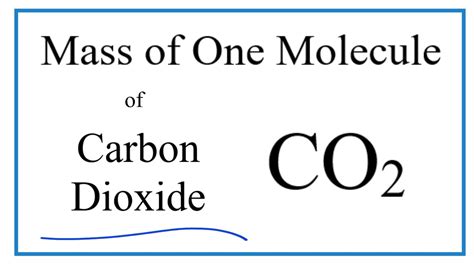
Table of Contents
Calculating the Molecular Mass of CO₂: A Comprehensive Guide
Determining the molecular mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This comprehensive guide delves into the process of calculating the molecular mass of carbon dioxide (CO₂), explaining the underlying principles and offering practical examples. We will also explore the significance of molecular mass in different chemical contexts.
Understanding Molecular Mass
Molecular mass, also known as molecular weight, represents the mass of a molecule. It's expressed in atomic mass units (amu) or Daltons (Da). Unlike molar mass, which refers to the mass of one mole of a substance (6.022 x 10²³ molecules), molecular mass focuses on the mass of a single molecule. The calculation involves summing the atomic masses of all atoms present in the molecule.
The Significance of Molecular Mass
Knowing the molecular mass of a compound is essential for various applications in chemistry and related fields:
- Stoichiometric Calculations: Molecular mass is fundamental in stoichiometry, allowing us to convert between mass and moles of a substance in chemical reactions.
- Gas Law Calculations: In ideal gas law calculations (PV=nRT), knowing the molecular mass helps determine the number of moles (n) of a gas given its mass.
- Solution Concentration: Calculating the concentration of a solution often requires knowledge of the solute's molecular mass.
- Spectroscopy: Molecular mass is used in interpreting mass spectrometry data, aiding in identifying unknown compounds.
- Thermodynamic Calculations: Understanding molecular mass aids in various thermodynamic calculations, such as enthalpy and entropy changes.
Calculating the Molecular Mass of CO₂
Carbon dioxide (CO₂) is a simple yet important molecule. Calculating its molecular mass requires knowledge of the atomic masses of carbon (C) and oxygen (O). These values can be found on the periodic table.
Atomic Masses from the Periodic Table
The periodic table provides the average atomic mass of each element, which is a weighted average of the isotopes of that element. For our calculation, we'll use standard values:
- Carbon (C): Approximately 12.01 amu
- Oxygen (O): Approximately 16.00 amu
Step-by-Step Calculation
-
Identify the atoms: CO₂ contains one carbon atom and two oxygen atoms.
-
Find atomic masses: From the periodic table, we obtain the atomic masses: Carbon (C) = 12.01 amu, Oxygen (O) = 16.00 amu.
-
Multiply by the number of atoms: Since there are two oxygen atoms, we multiply its atomic mass by 2: 2 x 16.00 amu = 32.00 amu.
-
Sum the atomic masses: Add the atomic mass of carbon and the total atomic mass of oxygen: 12.01 amu + 32.00 amu = 44.01 amu.
Therefore, the molecular mass of CO₂ is approximately 44.01 amu.
Practical Applications and Examples
Let's explore how the molecular mass of CO₂ is applied in real-world scenarios:
Example 1: Stoichiometry
Consider the combustion of methane (CH₄): CH₄ + 2O₂ → CO₂ + 2H₂O
If we have 16 grams of methane (CH₄), how many grams of CO₂ are produced?
-
Moles of CH₄: The molar mass of CH₄ is 16.04 g/mol. Therefore, 16g of CH₄ represents 1 mole.
-
Mole Ratio: From the balanced equation, 1 mole of CH₄ produces 1 mole of CO₂.
-
Mass of CO₂: Using the molecular mass of CO₂ (44.01 g/mol), 1 mole of CO₂ weighs 44.01 grams.
Therefore, 16 grams of CH₄ will produce 44.01 grams of CO₂.
Example 2: Ideal Gas Law
Suppose we have a sample of CO₂ gas with a volume of 2.5 liters at a temperature of 25°C (298 K) and a pressure of 1 atm. What is the mass of the CO₂ gas?
We will use the Ideal Gas Law: PV = nRT
Where:
- P = Pressure (1 atm)
- V = Volume (2.5 L)
- n = Number of moles
- R = Ideal gas constant (0.0821 L·atm/mol·K)
- T = Temperature (298 K)
Solving for n: n = PV/RT = (1 atm * 2.5 L) / (0.0821 L·atm/mol·K * 298 K) ≈ 0.102 moles
Then, using the molecular mass of CO₂ (44.01 g/mol), we calculate the mass:
Mass = n * Molecular Mass = 0.102 moles * 44.01 g/mol ≈ 4.49 grams
Example 3: Solution Concentration
Let's say we prepare a 0.1 M (molar) solution of CO₂ in water. To prepare 1 liter of this solution, how many grams of CO₂ are needed?
A 0.1 M solution means 0.1 moles of CO₂ per liter of solution.
Mass = n * Molecular Mass = 0.1 moles * 44.01 g/mol = 4.401 grams
Advanced Considerations
While the above calculations use average atomic masses from the periodic table, it's important to note that the actual mass of a CO₂ molecule can vary slightly due to the presence of different isotopes of carbon and oxygen. For highly precise calculations, isotopic abundances must be considered.
Conclusion
Calculating the molecular mass of CO₂ is a straightforward process that highlights the fundamental principles of chemistry. This seemingly simple calculation has wide-ranging applications in various fields, underscoring the importance of understanding molecular mass in chemical calculations and analyses. By mastering this fundamental concept, one gains a stronger foundation for tackling more complex chemical problems and interpreting experimental data. Remember to always utilize the most current and accurate atomic masses from a reliable periodic table for the most precise results.
Latest Posts
Latest Posts
-
Which Of The Following Is A Mineralocorticoid
Mar 25, 2025
-
Which Of The Following Is Not A Benefit Of Insurance
Mar 25, 2025
-
What Happens To The Plant Cell In A Hypertonic Solution
Mar 25, 2025
-
What Is 2000 Mg In Grams
Mar 25, 2025
-
The Code That A Programmer Writes Is Called Code
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Molecular Mass Of Co2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
