Calculate The Heat Of Combustion Of Ethene
News Leon
Mar 24, 2025 · 5 min read
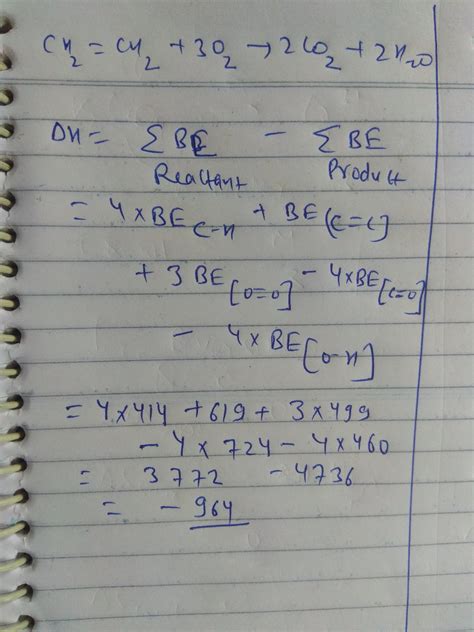
Table of Contents
Calculating the Heat of Combustion of Ethene: A Comprehensive Guide
Determining the heat of combustion, also known as the enthalpy of combustion, for a substance like ethene is crucial in various fields, from chemical engineering to environmental science. This value represents the amount of heat released when one mole of a substance undergoes complete combustion in the presence of oxygen. This article provides a comprehensive guide to calculating the heat of combustion of ethene (C₂H₄), exploring various methods and considerations.
Understanding Heat of Combustion
The heat of combustion is a measure of the energy stored within a chemical compound. It's an exothermic reaction, meaning it releases heat to the surroundings. For ethene, the combustion reaction is:
C₂H₄(g) + 3O₂(g) → 2CO₂(g) + 2H₂O(l)
This equation shows that one mole of ethene reacts with three moles of oxygen to produce two moles of carbon dioxide and two moles of water. The heat released during this process is the heat of combustion.
Methods for Calculating Heat of Combustion
There are several approaches to calculate the heat of combustion of ethene:
1. Using Standard Enthalpies of Formation (ΔfH°)
This is the most common and accurate method. It utilizes Hess's Law, which states that the enthalpy change of a reaction is independent of the pathway taken. We can calculate the heat of combustion using the standard enthalpies of formation of the reactants and products:
ΔcH° = Σ ΔfH°(products) - Σ ΔfH°(reactants)
Where:
- ΔcH° is the standard enthalpy change of combustion.
- ΔfH°(products) is the sum of the standard enthalpies of formation of the products (CO₂ and H₂O).
- ΔfH°(reactants) is the sum of the standard enthalpies of formation of the reactants (C₂H₄ and O₂).
Standard Enthalpies of Formation (kJ/mol):
- CO₂(g): -393.5
- H₂O(l): -285.8
- C₂H₄(g): +52.3
- O₂(g): 0 (by definition)
Applying Hess's Law:
ΔcH° = [2 * ΔfH°(CO₂(g)) + 2 * ΔfH°(H₂O(l))] - [ΔfH°(C₂H₄(g)) + 3 * ΔfH°(O₂(g))]
ΔcH° = [2 * (-393.5) + 2 * (-285.8)] - [52.3 + 3 * 0]
ΔcH° = (-787 - 571.6) - 52.3
ΔcH° = -1410.9 kJ/mol
This calculation indicates that the combustion of one mole of ethene releases approximately 1410.9 kJ of heat.
2. Using Bond Energies
This method is less precise than using standard enthalpies of formation but provides a reasonable estimate. It involves calculating the difference between the energy required to break the bonds in the reactants and the energy released when new bonds are formed in the products.
First, we need to identify the bonds present in the reactants and products:
Reactants:
- C₂H₄: 1 C=C bond (614 kJ/mol), 4 C-H bonds (413 kJ/mol each)
- 3O₂: 3 O=O bonds (498 kJ/mol each)
Products:
- 2CO₂: 4 C=O bonds (799 kJ/mol each)
- 2H₂O: 4 O-H bonds (467 kJ/mol each)
Calculation:
Energy required to break bonds in reactants:
(614 + 4413) + (3498) = 3266 kJ/mol
Energy released during bond formation in products:
(4799) + (4467) = 4980 kJ/mol
ΔcH° ≈ Energy released - Energy required = 4980 - 3266 = 1714 kJ/mol
The discrepancy between this value and the one obtained using standard enthalpies of formation highlights the limitations of the bond energy approach. Bond energies are average values, and actual bond energies can vary depending on the molecular environment.
3. Experimental Determination using Calorimetry
This is an experimental method that directly measures the heat released during the combustion of ethene. A calorimeter, a device designed to measure heat changes, is used. A known mass of ethene is burned in a controlled environment within the calorimeter, and the temperature change of the surrounding water is measured. Using the specific heat capacity of water and the mass of water, the heat released can be calculated.
This method requires careful calibration and control of experimental conditions to obtain accurate results. It is often considered the most reliable method, though it can be more complex and resource-intensive than the calculation methods discussed above.
Factors Affecting the Heat of Combustion
Several factors can influence the measured or calculated heat of combustion:
-
Phase of reactants and products: The physical state (solid, liquid, or gas) affects the enthalpy of combustion. The calculations above assume gaseous ethene and liquid water, which are typical conditions for combustion.
-
Temperature and pressure: The heat of combustion is usually reported at standard temperature and pressure (STP) conditions (298.15 K and 1 atm), but deviations from STP can affect the results.
-
Completeness of combustion: Incomplete combustion can lead to lower heat release as some carbon may be converted to carbon monoxide (CO) instead of carbon dioxide (CO₂).
-
Accuracy of data: Using inaccurate values for enthalpies of formation or bond energies will lead to inaccurate results.
Applications of Ethene Combustion Heat
Understanding the heat of combustion of ethene has numerous applications:
-
Fuel efficiency: In the context of fuel technologies, knowing the heat of combustion is critical in assessing the energy content and efficiency of ethene as a fuel source.
-
Chemical process design: In industrial chemical processes, this value is essential for designing efficient reactors and calculating energy requirements for various reactions.
-
Environmental impact assessment: The heat of combustion is crucial for determining the energy output and potential environmental consequences of ethene combustion, specifically regarding greenhouse gas emissions.
-
Thermochemical calculations: The heat of combustion serves as a vital parameter in broader thermochemical calculations, including equilibrium constants and reaction spontaneity.
Conclusion
Calculating the heat of combustion of ethene involves understanding the stoichiometry of the combustion reaction and employing appropriate methods. The most accurate method involves using standard enthalpies of formation and Hess's Law. While the bond energy method offers a simpler approach, it's less precise due to the inherent approximations in average bond energy values. Experimental calorimetric methods provide the most reliable results but require sophisticated equipment and procedures. Regardless of the method employed, understanding the potential influences on the measured or calculated value is critical for accurate interpretation and application of the heat of combustion of ethene. This value plays a significant role in diverse fields, making its accurate determination essential.
Latest Posts
Latest Posts
-
Uaa Uga And Uag Are All Codons
Mar 25, 2025
-
Sodium Is A Solid Liquid Or Gas
Mar 25, 2025
-
In The Figure Projectile Particle 1 Is An Alpha
Mar 25, 2025
-
Si Unit Of Measurement For Acceleration
Mar 25, 2025
-
What Is The Basic Unit For Distance
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Heat Of Combustion Of Ethene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
