Bond Order Of No In No3-
News Leon
Mar 26, 2025 · 5 min read
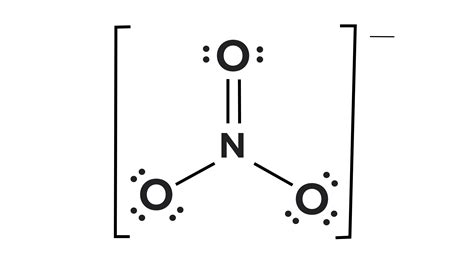
Table of Contents
Delving into the Bond Order of N-O Bonds in the Nitrate Ion (NO₃⁻)
The nitrate ion (NO₃⁻) is a ubiquitous polyatomic anion found in various chemical compounds and biological processes. Understanding its structure and bonding, particularly the bond order of the nitrogen-oxygen (N-O) bonds, is crucial for comprehending its reactivity and properties. This article delves deep into the intricacies of determining the bond order of N-O bonds within the NO₃⁻ ion, exploring different approaches and resolving apparent contradictions.
The Nitrate Ion Structure: A Resonance Hybrid
The nitrate ion exhibits a trigonal planar geometry, with the nitrogen atom at the center and three oxygen atoms surrounding it. A simple Lewis structure might suggest single and double bonds between the nitrogen and oxygen atoms, implying different bond lengths and strengths. However, this depiction is insufficient to accurately represent the actual bonding situation. Instead, the nitrate ion is best described as a resonance hybrid.
Resonance Structures and Delocalization
Three equivalent resonance structures can be drawn for NO₃⁻, where the double bond resonates among the three oxygen atoms. In each resonance structure, one N-O bond is a double bond, and the other two are single bonds.
(Diagram showing three resonance structures of NO3- would be inserted here. Due to markdown limitations, a textual representation is used instead.)
- Structure 1: N=O, N-O, N-O
- Structure 2: N-O, N=O, N-O
- Structure 3: N-O, N-O, N=O
The actual structure of the nitrate ion isn't any single one of these resonance structures; rather, it's a blend of all three. The electrons involved in the double bonds are delocalized across all three N-O bonds, resulting in an average bond order.
Calculating the Bond Order
The bond order is defined as the number of bonding electron pairs divided by the number of bonding positions. In the nitrate ion:
- Total number of valence electrons: N (5) + 3O (6 each) + 1 (negative charge) = 24 electrons.
- Electron distribution: A central nitrogen atom forms three sigma bonds with the three oxygen atoms, using six electrons. The remaining 18 electrons are distributed as six lone pairs on the oxygen atoms. However, to achieve a stable octet for nitrogen, we need to consider resonance.
The delocalization of electrons leads to an equalization of bond lengths and strengths. Therefore, a single Lewis structure is not representative; instead, we calculate the average bond order.
Average Bond Order Calculation
Given that each resonance structure contributes equally to the overall structure, the average bond order is calculated as follows:
For each N-O bond, we have:
- One double bond (bond order = 2) in one resonance structure.
- Two single bonds (bond order = 1) in two resonance structures.
The average bond order for each N-O bond in NO₃⁻ is therefore: (2 + 1 + 1) / 3 = 1.33
This means each N-O bond has a character intermediate between a single and a double bond, explaining the shorter than expected bond length observed experimentally.
Molecular Orbital Theory Perspective
While resonance structures provide a useful conceptual framework, molecular orbital (MO) theory offers a more rigorous quantum mechanical description of bonding. Applying MO theory to the nitrate ion reveals a more nuanced picture.
Sigma and Pi Bonding
The nitrate ion's bonding involves both sigma (σ) and pi (π) orbitals. The three σ bonds are formed by the overlap of nitrogen's sp² hybrid orbitals with the sp² hybrid orbitals of the oxygen atoms. The remaining p orbitals on nitrogen and oxygen participate in the formation of delocalized π molecular orbitals that extend over the entire ion.
Delocalized Pi System
These delocalized π orbitals lower the overall energy of the system, resulting in enhanced stability. This delocalization is responsible for the resonance effect observed in the Lewis structure approach. The electron density is evenly spread across the three N-O bonds, further supporting the 1.33 bond order.
Experimental Evidence Supporting the Bond Order
The predicted bond order of 1.33 for N-O bonds in NO₃⁻ is strongly supported by experimental data. X-ray crystallography and spectroscopic studies have consistently shown that all three N-O bond lengths are essentially equal, confirming the delocalization of electrons and the intermediate bond character.
Bond Length Measurements
The experimentally determined N-O bond length in nitrate salts aligns well with the predicted bond length for a bond order of approximately 1.33. It is shorter than a typical single bond but longer than a typical double bond, reflecting the partial double bond character.
Spectroscopic Data
Infrared (IR) and Raman spectroscopy provide further evidence of the delocalized bonding. The vibrational frequencies observed are consistent with the predicted values for a molecule with equal N-O bonds, further supporting the resonance hybrid model and the calculated bond order.
Implications of the Bond Order
The 1.33 bond order in NO₃⁻ has significant implications for its chemical reactivity and properties.
Enhanced Stability
The delocalized π system contributes significantly to the overall stability of the nitrate ion. This stability is reflected in its relatively low reactivity compared to molecules with localized double bonds.
Reactivity
Although relatively stable, the nitrate ion can still participate in various chemical reactions. The partial double bond character of the N-O bonds influences the reaction mechanisms and products formed. For instance, the nitrate ion can act as an oxidizing agent in certain reactions due to the availability of electrons in the delocalized π system.
Conclusion: A Unified Understanding
Determining the bond order of N-O bonds in NO₃⁻ requires considering both Lewis resonance structures and molecular orbital theory. The resonance hybrid model effectively captures the delocalization of electrons and the equal bond lengths observed experimentally. MO theory provides a deeper understanding of the underlying quantum mechanics and explains the enhanced stability due to the delocalized π system. The consistent experimental evidence, including bond length measurements and spectroscopic data, strongly supports the calculated average bond order of 1.33. This understanding is fundamental to comprehending the chemical behavior and reactivity of the nitrate ion in diverse chemical contexts. Further exploration into the intricacies of this seemingly simple ion continues to offer valuable insights into the complex world of chemical bonding.
Latest Posts
Latest Posts
-
What Is The Oxidation State Of Each Element In K2cr2o7
Mar 29, 2025
-
Which Of The Following Is An Unsaturated Fatty Acid
Mar 29, 2025
-
Match The Label To The Correct Structure On The Chloroplast
Mar 29, 2025
-
Whats The Difference Between Rough Er And Smooth Er
Mar 29, 2025
-
Dissolving Sugar In Water Physical Or Chemical Change
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Bond Order Of No In No3- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
