What Is The Oxidation State Of Each Element In K2cr2o7
News Leon
Mar 29, 2025 · 6 min read
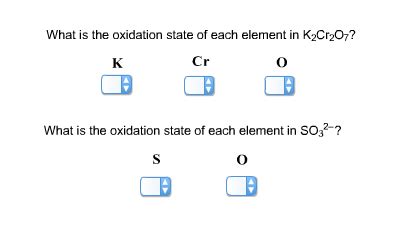
Table of Contents
What is the Oxidation State of Each Element in K₂Cr₂O₇?
Potassium dichromate, K₂Cr₂O₇, is a vibrant orange crystalline compound commonly used in various applications, from leather tanning to chemical synthesis. Understanding the oxidation states of its constituent elements – potassium (K), chromium (Cr), and oxygen (O) – is crucial for comprehending its chemical reactivity and properties. This article delves deep into determining the oxidation state of each element in K₂Cr₂O₇, explaining the concepts and methods involved.
Understanding Oxidation States
Before we embark on calculating the oxidation states in K₂Cr₂O₇, let's briefly review the fundamental concept of oxidation states. The oxidation state, also known as the oxidation number, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were completely ionic. It's a crucial tool in predicting the reactivity of compounds and balancing redox reactions. While not a true charge, it's a useful formalism for understanding electron transfer.
Several rules govern the assignment of oxidation states:
- Rule 1: The oxidation state of an element in its free (uncombined) state is always 0. For example, the oxidation state of elemental oxygen (O₂) is 0.
- Rule 2: The oxidation state of a monatomic ion is equal to its charge. For instance, the oxidation state of Na⁺ is +1 and Cl⁻ is -1.
- Rule 3: The oxidation state of hydrogen (H) is usually +1, except when bonded to less electronegative elements (like alkali metals and alkaline earth metals), where it is -1.
- Rule 4: The oxidation state of oxygen (O) is usually -2, except in peroxides (like H₂O₂), where it is -1, and in superoxides (like KO₂), where it is -1/2.
- Rule 5: The sum of the oxidation states of all atoms in a neutral molecule is 0.
- Rule 6: The sum of the oxidation states of all atoms in a polyatomic ion is equal to the charge of the ion.
Determining the Oxidation States in K₂Cr₂O₇
Now, let's apply these rules to determine the oxidation states of potassium, chromium, and oxygen in potassium dichromate (K₂Cr₂O₇).
Potassium (K)
Potassium is an alkali metal, belonging to Group 1 of the periodic table. Alkali metals readily lose one electron to achieve a stable noble gas configuration. Therefore, potassium consistently exhibits an oxidation state of +1.
Oxygen (O)
Oxygen is a highly electronegative element, and in most compounds, it exhibits an oxidation state of -2. Since there are no peroxides or superoxides present in K₂Cr₂O₇, we can safely assume the oxidation state of oxygen is -2.
Chromium (Cr)
Determining the oxidation state of chromium requires a systematic approach, utilizing the principles we've outlined.
-
Identify the known oxidation states: We know the oxidation state of potassium (+1) and oxygen (-2).
-
Apply the rule of charge neutrality: K₂Cr₂O₇ is a neutral molecule; therefore, the sum of the oxidation states of all its atoms must be zero.
-
Set up an algebraic equation: Let 'x' represent the oxidation state of chromium. We can construct the following equation:
2(+1) + 2(x) + 7(-2) = 0
-
Solve for x:
2 + 2x - 14 = 0 2x = 12 x = +6
Therefore, the oxidation state of chromium (Cr) in K₂Cr₂O₇ is +6.
Detailed Breakdown and Justification
Let's elaborate on why the +6 oxidation state for chromium is both logical and consistent with chemical principles:
-
Electron Configuration: Chromium's electron configuration is [Ar] 3d⁵ 4s¹. Achieving a +6 oxidation state involves the loss of all its valence electrons (the 4s and 3d electrons). This creates a stable, though not noble gas-like, configuration.
-
Chemical Bonding: The chromium atoms in K₂Cr₂O₇ are bonded to oxygen atoms through a network of covalent bonds with significant ionic character. The high electronegativity of oxygen effectively pulls electron density away from the chromium atoms, leading to the high positive oxidation state.
-
Dichromate Ion (Cr₂O₇²⁻): Understanding the dichromate ion itself provides further insight. The dichromate ion is a polyatomic anion with a charge of -2. The oxidation state calculation within the dichromate ion also leads to a +6 oxidation state for each chromium atom. The overall charge of -2 is balanced by the two potassium cations (K⁺).
Applications and Implications
The knowledge of oxidation states in K₂Cr₂O₇ has crucial implications in various fields:
-
Redox Reactions: Potassium dichromate is a strong oxidizing agent due to the high oxidation state of chromium (+6). This means it readily accepts electrons from other substances, causing them to be oxidized. This property is exploited in many redox titrations, where K₂Cr₂O₇ is used to determine the concentration of reducing agents.
-
Organic Chemistry: In organic chemistry, K₂Cr₂O₇ is used as an oxidizing agent in various reactions, such as the oxidation of alcohols to aldehydes or ketones. The +6 oxidation state of chromium makes it highly effective in these transformations.
-
Analytical Chemistry: The intense orange color of K₂Cr₂O₇ allows for its use in spectrophotometric analyses. Its properties as a strong oxidizing agent enable it to be used as an indicator in some titrations.
-
Industrial Applications: Aside from its use in leather tanning, K₂Cr₂O₇ finds applications in other industries, such as wood preservation, metal cleaning, and photography, where its oxidizing properties are exploited.
Safety Considerations
It's important to note that potassium dichromate is a hazardous substance. It's a strong oxidizing agent and can be irritating or corrosive to skin and eyes. Prolonged exposure may even lead to more serious health issues. Always handle K₂Cr₂O₇ with appropriate safety precautions, including wearing protective gloves and eye protection, working in a well-ventilated area, and following proper disposal procedures.
Conclusion
In conclusion, the oxidation states of the elements in K₂Cr₂O₇ are: K (+1), Cr (+6), and O (-2). Understanding these oxidation states is fundamental to understanding the chemical behavior and reactivity of potassium dichromate, its use in various applications, and the safety precautions associated with its handling. This knowledge is vital for students of chemistry, professionals working in related fields, and anyone seeking to appreciate the intricate world of chemical compounds. Further exploration of redox chemistry and the principles governing oxidation states will enhance understanding of this important chemical compound and its properties. The calculation method demonstrated here serves as a general model applicable to many other compounds. By mastering this fundamental principle, you'll be better equipped to tackle more complex chemical scenarios. Remember always to prioritize safety when working with chemicals.
Latest Posts
Latest Posts
-
How Many Isotopes Does Fluorine Have
Mar 31, 2025
-
Refraction Causes The Bottom Of A Swimming Pool To Appear
Mar 31, 2025
-
Glucagon Stimulates Glycogenolysis In The Liver True Or False
Mar 31, 2025
-
Classify The Following Mixtures As Heterogeneous Or Homogeneous
Mar 31, 2025
-
Is Steel A Homogeneous Or Heterogeneous Mixture
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation State Of Each Element In K2cr2o7 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
