Boiling Water Is A Chemical Change
News Leon
Mar 29, 2025 · 5 min read
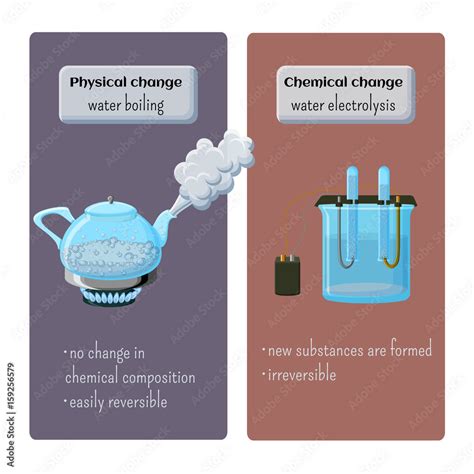
Table of Contents
Is Boiling Water a Chemical Change? A Deep Dive into the Science
The question of whether boiling water represents a chemical change or a physical change is a surprisingly complex one, often sparking debate among science enthusiasts and students alike. While seemingly straightforward, a thorough understanding requires a careful examination of the definitions of chemical and physical changes, along with a detailed look at the molecular transformations occurring during the boiling process. This article will delve deep into the science, exploring the nuances of this seemingly simple phenomenon.
Understanding Chemical vs. Physical Changes
Before tackling the boiling water conundrum, let's establish a clear understanding of the fundamental differences between chemical and physical changes.
Physical changes alter the form or appearance of a substance but do not change its chemical composition. Think about cutting paper, melting ice, or dissolving sugar in water. In each case, the substance's chemical identity remains unchanged. It's merely a shift in its physical state or form. The original substance can be recovered through simple physical processes like freezing, evaporation, or filtration.
Chemical changes, on the other hand, involve the formation of new substances with different chemical properties. These changes are often accompanied by observable signs like a color change, gas production, precipitation (formation of a solid), or a significant temperature change. Crucially, the original substance cannot be easily recovered through simple physical means; a chemical reaction has altered its fundamental nature. Examples include burning wood, rusting iron, or baking a cake.
Analyzing the Boiling Process: A Molecular Perspective
Let's scrutinize what happens when water boils. Water, in its liquid state (H₂O), exists as a collection of water molecules held together by relatively weak intermolecular forces (hydrogen bonds). These bonds are responsible for water's unique properties, such as its high boiling point.
When heat is applied, the kinetic energy of the water molecules increases. This increased energy allows the molecules to overcome the intermolecular forces holding them together in the liquid phase. As the temperature reaches 100°C (at standard atmospheric pressure), the water begins to boil. The molecules with sufficient kinetic energy escape the liquid phase and transition into the gaseous phase (steam or water vapor).
The key takeaway here is that the water molecules themselves remain unchanged. Each molecule still consists of two hydrogen atoms and one oxygen atom (H₂O). No new chemical bonds are formed, and no existing chemical bonds are broken. The transformation is solely a change in the state of matter, from liquid to gas.
The Argument for a Physical Change
Based on the molecular-level analysis, the overwhelming scientific consensus classifies boiling water as a physical change. The process involves only a change in the state of matter, driven by the increased kinetic energy of the water molecules overcoming intermolecular forces. The chemical identity of the water molecules remains intact throughout the entire process. By condensing the steam back into liquid water, we effectively reverse the process, demonstrating the reversibility characteristic of a physical change.
Addressing Counterarguments and Nuances
While the primary conclusion points towards a physical change, some might argue that the high temperatures involved could trigger minor chemical reactions, such as the dissociation of some water molecules into hydrogen and oxygen ions (H⁺ and OH⁻). This process, known as autoionization of water, does occur to a very limited extent even at room temperature. However, the concentration of these ions is extremely low and does not significantly alter the overall chemical composition of the water. The amount of dissociation is negligible compared to the total number of water molecules present.
Furthermore, the presence of impurities in the water could lead to some minor chemical reactions during boiling. For instance, dissolved minerals might precipitate out of solution or undergo slight chemical alteration. However, these side reactions are secondary and do not fundamentally change the nature of the boiling process itself. The main transformation—the phase transition from liquid to gas—remains a physical change.
In essence, these minor chemical reactions are insignificant compared to the dominant physical change of phase transition. They are more accurately considered side effects rather than the primary characteristic of the boiling process.
The Importance of Context in Scientific Classifications
It's important to acknowledge that the classification of a process as either a chemical or physical change can be context-dependent. In a highly controlled laboratory setting, with extremely pure water and rigorous analysis, one might detect traces of chemical reactions associated with boiling. However, for practical purposes and in everyday life, boiling water is undeniably a physical change. The transformation is overwhelmingly dominated by the phase transition, and the minor chemical side effects are negligible.
Practical Implications and Conclusion
Understanding the distinction between physical and chemical changes is vital in various scientific fields, including chemistry, physics, and engineering. In the case of boiling water, this understanding helps us to predict and control various processes, such as steam generation for power plants or sterilization in food processing.
In conclusion, while extremely subtle chemical reactions might accompany the boiling of water, the dominant process is undoubtedly a physical change. The phase transition from liquid to gas is the defining characteristic of boiling water, and the chemical identity of the water molecule remains unchanged. The minor chemical side effects are negligible compared to this primary physical transformation. Therefore, for all practical purposes, boiling water is accurately classified as a physical change. This clarification underscores the importance of analyzing processes at the molecular level to accurately categorize them within the context of chemical and physical transformations.
Latest Posts
Latest Posts
-
If The Cross Product Of Two Vectors Is Zero
Mar 31, 2025
-
How To Use Insert In Python
Mar 31, 2025
-
How Many Lines Of Symmetry Does Scalene Triangle Have
Mar 31, 2025
-
What Is The Ultimate Purpose Of A Political Party
Mar 31, 2025
-
What Is The Smallest Unit Of Dna Called
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Is A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
