Another Name For A Dehydration Reaction Is A Reaction.
News Leon
Mar 28, 2025 · 6 min read
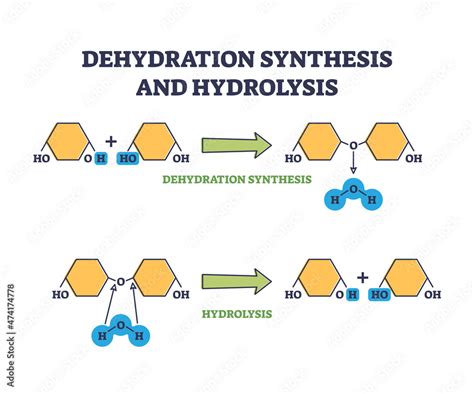
Table of Contents
Another Name for a Dehydration Reaction is a Condensation Reaction
Dehydration reactions are fundamental processes in organic chemistry and biochemistry, playing crucial roles in the synthesis of a vast array of molecules, from simple sugars to complex polymers. Understanding these reactions is key to comprehending many biological processes and industrial chemical syntheses. While often referred to as dehydration reactions, they are more accurately and comprehensively described as condensation reactions. This article will delve deep into the intricacies of dehydration reactions, exploring their mechanisms, applications, and the critical distinction between the terms "dehydration" and "condensation".
Dehydration Reactions: Losing Water to Gain Bonds
A dehydration reaction, more appropriately termed a condensation reaction, is a chemical reaction in which two molecules combine to form a larger molecule, with the simultaneous loss of a small molecule, most commonly water. The process involves the removal of a hydroxyl group (-OH) from one molecule and a hydrogen atom (-H) from another, forming a water molecule (H₂O) and creating a new covalent bond between the two original molecules. This newly formed bond links the two molecules together, resulting in a larger, more complex molecule.
Mechanism of Dehydration Reactions
The mechanism of a dehydration reaction typically involves several steps:
-
Protonation: A proton (H⁺) from a catalyst, often an acid, is added to one of the reacting molecules, usually an alcohol or a carboxylic acid. This protonation increases the reactivity of the molecule.
-
Departure of a Leaving Group: A water molecule leaves, acting as a leaving group. This step forms a carbocation intermediate, a highly reactive species with a positively charged carbon atom.
-
Nucleophilic Attack: Another molecule, often another alcohol or a nucleophile, attacks the carbocation. This attack forms a new covalent bond.
-
Deprotonation: A proton is removed from the newly formed molecule, resulting in the final product.
The specific steps and intermediates can vary depending on the reactants and reaction conditions, but the overall process of water removal and bond formation remains consistent.
Examples of Dehydration Reactions
Dehydration reactions are ubiquitous in organic chemistry and biology. Here are some prominent examples:
-
Esterification: The reaction between a carboxylic acid and an alcohol produces an ester and water. This reaction is commonly used in the synthesis of perfumes, flavors, and pharmaceuticals.
-
Formation of Peptides: The formation of peptide bonds between amino acids to form proteins is a classic example of a dehydration reaction. The carboxyl group of one amino acid reacts with the amino group of another, releasing a water molecule and forming a peptide bond (amide bond).
-
Formation of Glycosidic Bonds: The formation of glycosidic bonds between monosaccharides to create disaccharides and polysaccharides is another important dehydration reaction. This process is vital for carbohydrate metabolism and storage.
-
Dehydration of Alcohols: Alcohols can undergo dehydration to form alkenes. This reaction often requires the presence of a strong acid catalyst, such as sulfuric acid.
-
Dehydration Synthesis of Polymers: Many synthetic polymers are made through dehydration reactions, where repeating monomer units are joined together by the removal of water molecules. This is a crucial process in the production of plastics and other synthetic materials.
The Importance of the Term "Condensation Reaction"
While "dehydration reaction" accurately describes the loss of water, the term "condensation reaction" provides a more comprehensive and accurate description of the overall process. This is because:
-
Focus on Bond Formation: "Condensation reaction" emphasizes the key event – the formation of a new covalent bond between two molecules. This is a more fundamental aspect of the reaction than simply the loss of water.
-
Broader Applicability: The term "condensation reaction" encompasses a wider range of reactions where a small molecule is eliminated, not just water. Other small molecules, such as methanol or ammonia, can also be eliminated during the formation of larger molecules. Using "condensation reaction" avoids limiting the description to only water-loss reactions.
-
Improved Clarity: Using "condensation reaction" avoids potential confusion with other chemical processes that might also involve water loss. For example, the removal of water from a hydrate is different from the joining of two molecules with water loss. "Condensation reaction" clearly describes the joining of molecules.
Distinguishing Dehydration and Condensation: A Closer Look
The terms "dehydration reaction" and "condensation reaction" are often used interchangeably, especially in introductory chemistry courses. However, a nuanced understanding recognizes "condensation reaction" as the broader, more encompassing term. A dehydration reaction is a type of condensation reaction, specifically one where water is the small molecule eliminated.
Imagine the analogy of a family tree. "Condensation reaction" represents the larger family, while "dehydration reaction" represents a specific branch within that family. All dehydration reactions are condensation reactions, but not all condensation reactions are dehydration reactions.
Therefore, using "condensation reaction" offers greater precision and avoids the limitations inherent in the narrower term "dehydration reaction".
Applications of Dehydration/Condensation Reactions in Various Fields
The significance of dehydration/condensation reactions extends far beyond the realm of theoretical chemistry. Their applications are pervasive across numerous fields:
Biochemistry and Biology
-
Protein Synthesis: As mentioned earlier, the creation of peptide bonds during protein synthesis relies on a condensation reaction. This fundamental process is critical for life itself.
-
Carbohydrate Metabolism: The formation and breakdown of carbohydrates, including sugars and starches, involve dehydration and hydrolysis (the reverse of condensation) reactions.
-
Nucleic Acid Synthesis: The polymerization of nucleotides to form DNA and RNA strands employs condensation reactions, linking nucleotides through phosphodiester bonds.
-
Lipid Metabolism: The synthesis of lipids and fats involves condensation reactions, joining fatty acids to glycerol.
Industrial Applications
-
Polymer Synthesis: The production of numerous synthetic polymers, including nylon, polyester, and many plastics, relies heavily on condensation polymerization. These polymers form the backbone of countless consumer products.
-
Pharmaceutical Industry: Many drugs and pharmaceuticals are synthesized using condensation reactions to create complex molecules with specific biological activities.
-
Food Industry: The production of certain food items, like some types of candy and processed foods, employs dehydration/condensation reactions to alter the properties of the ingredients.
Other Applications
Condensation reactions also find applications in:
-
Materials Science: The creation of novel materials with specific properties can be achieved through tailored condensation reactions.
-
Environmental Science: Understanding condensation reactions is important for analyzing the formation and breakdown of various compounds in the environment.
-
Forensic Science: The analysis of condensation reactions can be helpful in forensic investigations.
Conclusion: Understanding the Nuances for Better Comprehension
In summary, while the terms "dehydration reaction" and "condensation reaction" are frequently used synonymously, a more precise understanding reveals "condensation reaction" as the overarching and more accurate term. Dehydration reactions are a subset of condensation reactions, specifically those where water is the small molecule eliminated. This distinction is crucial for a comprehensive understanding of these reactions and their vast applications across diverse scientific and industrial fields. Understanding the nuanced relationship between these terms enhances scientific communication and fosters a deeper appreciation for the fundamental role these reactions play in shaping the world around us. By utilizing the more comprehensive term "condensation reaction," we achieve greater clarity, accuracy, and a broader scope of understanding within the field of chemistry. The importance of these reactions cannot be overstated, from the building blocks of life itself to the creation of countless synthetic materials that shape our modern world. Future research and advancements in understanding condensation reactions will undoubtedly lead to further breakthroughs in various scientific and technological domains.
Latest Posts
Latest Posts
-
True Or False Evaporation Is A Physical Change
Mar 31, 2025
-
Do Gram Positive Bacteria Have Porins
Mar 31, 2025
-
Which Of The Following Compounds Is Most Soluble In Water
Mar 31, 2025
-
Part Of The Brain That Controls Breathing And Heartbeat
Mar 31, 2025
-
This Pair Of Structures Anchors The Spindle
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Another Name For A Dehydration Reaction Is A Reaction. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
