An Atom That Has Gained An Electron Is Called
News Leon
Apr 07, 2025 · 6 min read
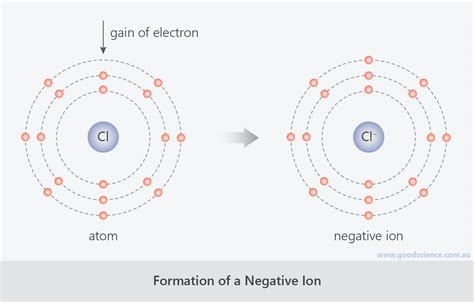
Table of Contents
An Atom That Has Gained an Electron Is Called an Anion: A Deep Dive into Ionic Bonds and Chemical Reactions
Have you ever wondered what happens when an atom, the fundamental building block of matter, acquires an extra electron? The answer is surprisingly impactful and forms the basis of many chemical reactions and the properties of countless materials. An atom that has gained an electron is called an anion. This seemingly simple process leads to a cascade of effects, fundamentally altering the atom's electrical charge and its interactions with other atoms. This article will explore the concept of anions in detail, delving into their formation, properties, and crucial role in chemistry.
Understanding Atomic Structure and Electron Configuration
Before diving into the specifics of anion formation, it's crucial to understand the basic structure of an atom. Atoms consist of a central nucleus containing positively charged protons and neutral neutrons, surrounded by negatively charged electrons orbiting in specific energy levels or shells. The number of protons determines the element's atomic number and its identity, while the number of electrons determines its charge and reactivity.
Atoms strive for stability, often seeking a full outermost electron shell. This is explained by the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight electrons in their valence shell (the outermost electron shell). Exceptions exist, particularly for elements in the first and second rows of the periodic table, which may achieve stability with two electrons in their outermost shell.
Valence Electrons and Chemical Reactivity
Valence electrons, located in the outermost shell, are the primary players in chemical bonding. These electrons are loosely held and readily participate in interactions with other atoms. Atoms with nearly full valence shells are more likely to gain electrons, while those with few valence electrons are more inclined to lose them.
The Formation of Anions: Gaining Electrons for Stability
When an atom gains one or more electrons, it acquires a net negative charge because the number of negatively charged electrons now exceeds the number of positively charged protons. This negatively charged atom is now called an anion. The process of anion formation is a key aspect of ionic bonding, a type of chemical bond formed through the electrostatic attraction between oppositely charged ions.
The strength of the attraction between the anion and a positively charged cation (an atom that has lost electrons) depends on several factors including the charges of the ions and the distance between them. Larger charges lead to stronger attractions, while greater distances weaken the attraction.
Examples of Anion Formation
Let's consider a few examples to illustrate anion formation:
-
Chlorine (Cl): Chlorine has seven valence electrons. To achieve a stable octet, it readily gains one electron, forming a chloride anion (Cl⁻). This is a highly common anion in many compounds.
-
Oxygen (O): Oxygen has six valence electrons. It gains two electrons to achieve a stable octet, forming an oxide anion (O²⁻). Oxide anions are essential components of many minerals and compounds.
-
Sulfur (S): Sulfur, like oxygen, has six valence electrons and typically gains two electrons to form a sulfide anion (S²⁻). Sulfide anions are found in many metal sulfides.
-
Nitrogen (N): Nitrogen has five valence electrons and often gains three electrons to form a nitride anion (N³⁻). Nitrides are often found in materials with unique properties.
Properties of Anions
Anions exhibit several distinct properties stemming from their negative charge:
-
Electrostatic Attraction: Anions are attracted to cations due to their opposite charges. This electrostatic attraction is the driving force behind ionic bonding.
-
Solubility: The solubility of ionic compounds, which are formed by anions and cations, varies greatly depending on the specific ions involved and the solvent. Many ionic compounds are soluble in polar solvents like water, where the polar water molecules can interact with and stabilize the charged ions.
-
Electrical Conductivity: Ionic compounds in their molten state or dissolved in a suitable solvent conduct electricity because the mobile ions can carry an electric current.
-
Crystal Structure: Ionic compounds often form crystalline structures, where the anions and cations are arranged in a regular, repeating pattern to maximize electrostatic attraction and minimize repulsion. The specific crystal structure depends on the sizes and charges of the ions involved.
Anions in Chemical Reactions and Biological Systems
Anions play a crucial role in a wide array of chemical reactions and biological processes. Their involvement in these processes stems from their ability to participate in ionic bonding, donate electrons in redox reactions, and act as ligands in coordination complexes.
Anions and Redox Reactions
Anions can participate in redox reactions, which involve the transfer of electrons between chemical species. In these reactions, anions can act as either oxidizing agents (accepting electrons) or reducing agents (donating electrons), depending on the specific reaction and the other reactants involved.
Anions in Biological Systems
Anions are essential components of biological systems. Many vital biological molecules, including proteins, nucleic acids, and carbohydrates, contain anionic groups. These anionic groups are crucial for the functions of these molecules, such as enzyme activity, protein folding, and DNA structure.
Examples of biologically important anions include:
- Phosphate (PO₄³⁻): A crucial component of ATP (adenosine triphosphate), the energy currency of cells.
- Bicarbonate (HCO₃⁻): Plays a vital role in maintaining blood pH.
- Chloride (Cl⁻): Important for maintaining fluid balance and nerve impulse transmission.
The Importance of Anions in Materials Science
Anions are indispensable in materials science. Their properties directly influence the characteristics of numerous materials, including:
-
Ceramics: Many ceramic materials are based on ionic compounds containing various anions, such as oxides, nitrides, and carbides. The properties of these ceramics, such as hardness, strength, and thermal stability, are directly related to the types and arrangements of anions present.
-
Semiconductors: Anions can be incorporated into semiconductor materials to modify their electronic properties. Doping semiconductors with specific anions can alter their conductivity, enabling the creation of transistors and other semiconductor devices.
-
Batteries: Many battery systems utilize anions as part of the electrolyte or cathode materials. The properties of the anions, such as their mobility and reactivity, significantly impact the performance of the battery.
Distinguishing Anions from other Ions
It's important to clearly distinguish anions from other types of ions. While anions are negatively charged, cations are positively charged. The difference in charge leads to different chemical behaviors and interactions. Understanding this distinction is key to predicting chemical reactions and understanding material properties.
Conclusion: The Ubiquitous Role of Anions
In conclusion, an atom that has gained an electron is called an anion. This seemingly simple process has profound consequences, leading to significant changes in the atom's charge, reactivity, and its interactions with other atoms and molecules. Anions are integral to various processes, from simple chemical reactions to complex biological mechanisms and material properties. Their presence and characteristics fundamentally shape the world around us, influencing the materials we use, the reactions we observe, and the life processes that occur within us. A comprehensive understanding of anions is therefore critical for advancing our knowledge in chemistry, biology, materials science, and many other scientific disciplines.
Latest Posts
Latest Posts
-
5 Letter Word With 3 Vowels And 2 Consonants
Apr 08, 2025
-
Is H2 O2 H2o A Redox Reaction
Apr 08, 2025
-
Which Of The Following Can Serve As A Nucleophile
Apr 08, 2025
-
Oxidation Number Of S In So3
Apr 08, 2025
-
Replication Is Called Semi Conservative Because
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about An Atom That Has Gained An Electron Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.