Which Of The Following Can Serve As A Nucleophile
News Leon
Apr 08, 2025 · 5 min read
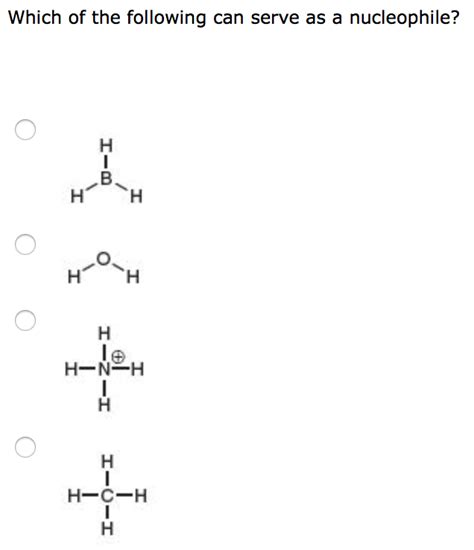
Table of Contents
Which of the Following Can Serve as a Nucleophile?
Understanding nucleophiles is crucial in organic chemistry. A nucleophile, literally meaning "nucleus-loving," is a chemical species that donates an electron pair to an electrophile, an electron-deficient species, to form a chemical bond. This electron donation forms the basis of many important reactions, including substitution, addition, and elimination reactions. But not all chemical species possess the capability to act as nucleophiles. This article explores the criteria that determine nucleophilicity and examines various chemical species to determine their nucleophilic potential.
Defining Nucleophilicity: More Than Just a Lone Pair
While the presence of a lone pair of electrons is a prerequisite for nucleophilicity, it's not the sole determinant. Several factors influence a species' nucleophilicity:
1. Charge:
Negatively charged species are generally stronger nucleophiles than neutral species. The extra electron density increases their ability to donate electrons. For example, hydroxide ion (OH⁻) is a stronger nucleophile than water (H₂O). The negative charge on the oxygen atom significantly enhances its electron-donating ability.
2. Electronegativity:
Lower electronegativity equates to stronger nucleophilicity. Electronegativity measures an atom's tendency to attract electrons. Less electronegative atoms hold their electrons less tightly, making them more readily available for donation. For instance, sulfur (S) is a better nucleophile than oxygen (O) because it's less electronegative.
3. Size/Steric Hindrance:
Smaller nucleophiles generally react faster than larger ones. This is primarily due to steric hindrance. Bulky nucleophiles have difficulty approaching the electrophilic center, thus slowing down the reaction. However, this effect can be context-dependent; sometimes, larger nucleophiles can be better due to better solvation or other factors.
4. Solvent Effects:
The solvent plays a significant role in nucleophilicity. Protic solvents (those with O-H or N-H bonds) solvate anions effectively, reducing their nucleophilicity. This is because the solvent molecules surround the anion, hindering its ability to approach the electrophile. Aprotic solvents (without O-H or N-H bonds) have less of an impact on nucleophilicity. Therefore, the same nucleophile can exhibit different nucleophilicity in different solvents.
Identifying Potential Nucleophiles: Case Studies
Let's analyze several chemical species and assess their nucleophilic capabilities based on the criteria outlined above:
1. Water (H₂O):
Water possesses two lone pairs of electrons on the oxygen atom. However, its relatively high electronegativity and neutral charge make it a weak nucleophile. It participates in nucleophilic reactions, but usually only under specific conditions.
2. Hydroxide Ion (OH⁻):
The hydroxide ion carries a negative charge and possesses lone pairs on the oxygen atom. The negative charge significantly boosts its electron-donating ability. Compared to water, hydroxide is a much stronger nucleophile.
3. Ammonia (NH₃):
Ammonia has a lone pair of electrons on the nitrogen atom. While neutral, its less electronegative nitrogen atom makes it a better nucleophile than water. Ammonia is a moderate nucleophile, commonly used in various reactions.
4. Chloride Ion (Cl⁻):
The chloride ion is a negatively charged species with lone pairs of electrons. Its size is larger compared to hydroxide, potentially leading to some steric hindrance. However, the negative charge makes it a reasonably good nucleophile. The strength of its nucleophilicity is affected by the solvent.
5. Bromide Ion (Br⁻):
Similar to chloride, the bromide ion is negatively charged and has lone pairs. It's larger than chloride, resulting in increased steric hindrance. However, its less electronegative nature compared to chloride makes it a better nucleophile than chloride in many aprotic solvents.
6. Iodide Ion (I⁻):
Iodide, being the largest of the halide ions, experiences significant steric hindrance. However, its low electronegativity and negative charge counteract this effect to some extent. In aprotic solvents, iodide is often considered the strongest nucleophile amongst the halides.
7. Thiols (RSH):
Thiols contain a sulfur atom bonded to a hydrogen atom. Sulfur is significantly less electronegative than oxygen, making the lone pair on sulfur much more readily available for donation. Thiols are generally stronger nucleophiles than their corresponding alcohols (ROH).
8. Cyanide Ion (CN⁻):
Cyanide is a negatively charged species with a lone pair of electrons on the carbon atom. The carbon atom is less electronegative than nitrogen, making the carbon the primary nucleophilic center. Cyanide is a powerful nucleophile often used in substitution and addition reactions.
9. Grignard Reagents (RMgX):
Grignard reagents are organomagnesium halides, where the carbon atom bonded to magnesium possesses significant nucleophilic character. The carbon atom bears a partial negative charge due to the electronegativity difference between carbon and magnesium. Grignard reagents are extremely strong nucleophiles frequently utilized to form new carbon-carbon bonds.
10. Organolithium Reagents (RLi):
Similar to Grignard reagents, organolithium compounds are very strong nucleophiles. The carbon atom bonded to lithium carries a significant partial negative charge, enhancing its nucleophilicity. These are powerful reagents often employed in organic synthesis.
Factors Influencing Nucleophile Strength: A Deeper Dive
The relative nucleophilicity of different species can vary significantly depending on the reaction conditions. Let's explore some key factors that affect the observed nucleophilicity:
-
Leaving group ability: A good leaving group facilitates the reaction by readily departing from the electrophile. A weaker leaving group can hinder the reaction even if a strong nucleophile is present.
-
Substrate sterics: Steric hindrance around the electrophilic center can affect the approach of the nucleophile. Bulky substrates may hinder the reaction even with a strong nucleophile.
-
Temperature: Higher temperatures generally increase reaction rates, including nucleophilic reactions.
-
Concentration: Increased concentration of the nucleophile often leads to faster reaction rates.
-
Solvent effects: As discussed earlier, the choice of solvent critically influences the solvation of the nucleophile and, consequently, its reactivity. Protic solvents often reduce nucleophilicity, while aprotic solvents tend to enhance it.
Conclusion: Context Matters
Determining whether a given chemical species can act as a nucleophile involves considering multiple factors: charge, electronegativity, size, and solvent effects. While some species, like Grignard reagents, are consistently potent nucleophiles, others, like water, exhibit weak nucleophilicity under most conditions. The effectiveness of a nucleophile is highly context-dependent, meaning the reaction conditions and the specific substrate greatly influence its behavior. Therefore, a thorough understanding of these factors is essential for predicting and controlling the outcome of nucleophilic reactions in organic chemistry. Remember, the ability to effectively analyze and predict nucleophilic behavior is a cornerstone of successful organic synthesis.
Latest Posts
Latest Posts
-
A Mixture In Which The Composition Is Uniform Throughout
Apr 08, 2025
-
Is Carbon Dioxide A Covalent Bond
Apr 08, 2025
-
What Happens When A Star Exhausts Its Core Hydrogen Supply
Apr 08, 2025
-
Find The Value Of X In A Kite
Apr 08, 2025
-
Which Of The Following Is True Regarding The Sarcoplasmic Reticulum
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Can Serve As A Nucleophile . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.