Oxidation Number Of S In So3
News Leon
Apr 08, 2025 · 6 min read
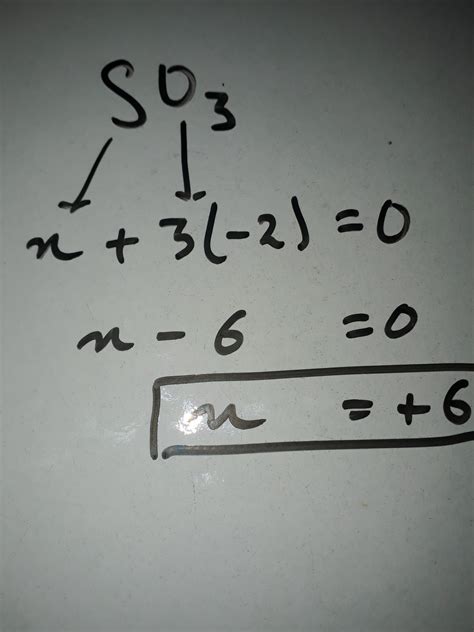
Table of Contents
Determining the Oxidation Number of Sulfur in SO₃
Sulfur trioxide (SO₃), a crucial intermediate in the production of sulfuric acid, presents an interesting case study in understanding oxidation numbers. This article delves deep into the methodology of calculating the oxidation number of sulfur in SO₃, exploring the underlying principles and providing a comprehensive understanding of the concept within the broader context of chemical bonding and reactivity. We'll also explore the implications of this oxidation state for SO₃'s properties and its role in various chemical reactions.
Understanding Oxidation Numbers
Before we jump into the calculation for SO₃, let's establish a firm foundation. The oxidation number, also known as the oxidation state, is a number assigned to an atom in a chemical compound that represents the number of electrons that atom has gained or lost compared to its neutral state. It's a crucial concept in chemistry for balancing redox reactions, predicting reactivity, and understanding the electronic structure of compounds.
Key Rules for Assigning Oxidation Numbers:
- Free elements: The oxidation number of an atom in its elemental form is always 0. For example, the oxidation number of S in S₈ is 0.
- Monatomic ions: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
- Hydrogen: Hydrogen usually has an oxidation number of +1, except in metal hydrides (e.g., NaH) where it's -1.
- Oxygen: Oxygen usually has an oxidation number of -2, except in peroxides (e.g., H₂O₂) where it's -1 and in compounds with fluorine (e.g., OF₂) where it's +2.
- Fluorine: Fluorine always has an oxidation number of -1.
- The sum of oxidation numbers: In a neutral compound, the sum of the oxidation numbers of all atoms must equal zero. In a polyatomic ion, the sum of the oxidation numbers must equal the charge of the ion.
Calculating the Oxidation Number of Sulfur in SO₃
Now, let's apply these rules to determine the oxidation number of sulfur (S) in sulfur trioxide (SO₃).
Step 1: Identify the oxidation numbers of the other elements.
- Oxygen (O) typically has an oxidation number of -2. There are three oxygen atoms in SO₃.
Step 2: Set up an equation based on the sum of oxidation numbers.
Let 'x' represent the oxidation number of sulfur (S). Since SO₃ is a neutral molecule, the sum of the oxidation numbers must equal zero. Therefore, we can write the equation:
x + 3(-2) = 0
Step 3: Solve for x.
x - 6 = 0 x = +6
Therefore, the oxidation number of sulfur (S) in SO₃ is +6.
Implications of the +6 Oxidation State
The +6 oxidation state of sulfur in SO₃ has significant implications for its chemical properties and reactivity:
-
Strong Oxidizing Agent: Sulfur in the +6 oxidation state is highly oxidized and acts as a strong oxidizing agent. It readily accepts electrons from other substances, leading to reduction reactions. This is a key reason why SO₃ is a crucial intermediate in the production of sulfuric acid (H₂SO₄), a powerful oxidizing agent itself.
-
Acidic Nature: SO₃ reacts vigorously with water to form sulfuric acid (H₂SO₄), a strong acid. This acidic nature is directly related to the high oxidation state of sulfur, which makes the molecule highly polar and capable of donating protons (H⁺).
-
Reactivity with Bases: Because of its acidic nature, SO₃ reacts readily with bases, forming sulfates. This reaction is a neutralization reaction where the acidic SO₃ reacts with a base to form a salt (sulfate) and water.
-
Role in Industrial Processes: The +6 oxidation state of sulfur in SO₃ highlights its importance in industrial processes. It's a key intermediate in the contact process, the primary industrial method for producing sulfuric acid, one of the most important industrial chemicals globally. Its high reactivity and ability to act as an oxidizing agent are crucial to its role in this process.
Comparison with Other Sulfur Oxidation States
It's helpful to compare the +6 oxidation state of sulfur in SO₃ to other possible oxidation states of sulfur. Sulfur can exhibit a wide range of oxidation states, including -2, 0, +2, +4, and +6. The oxidation state significantly influences the chemical behavior of the sulfur compound:
-
-2 (Sulfides): In sulfides (e.g., H₂S), sulfur exhibits a -2 oxidation state, acting as a reducing agent. These compounds are typically characterized by their pungent odor and are significantly less reactive than those with higher oxidation states.
-
0 (Elemental Sulfur): Elemental sulfur (S₈) has an oxidation state of 0 and is relatively unreactive compared to its oxidized forms.
-
+2 (Sulfur Dioxide): Sulfur dioxide (SO₂) has sulfur in the +2 oxidation state. It's a weaker oxidizing agent than SO₃ and exhibits different chemical properties, including its use as a preservative and disinfectant.
-
+4 (Sulfurous Acid): Sulfurous acid (H₂SO₃) contains sulfur with a +4 oxidation state. It is a weaker acid compared to sulfuric acid and is less stable.
Advanced Concepts and Applications
The understanding of the oxidation number of sulfur in SO₃ extends beyond basic stoichiometry. More advanced concepts include:
-
Redox Reactions: The +6 oxidation state of sulfur is crucial in understanding the redox reactions involving SO₃. Its ability to act as an oxidizing agent is central to many industrial processes and naturally occurring chemical reactions.
-
Electrochemistry: The oxidation state is relevant to electrochemistry, specifically in electrochemical cells and the prediction of electrode potentials. The high oxidation state suggests a high tendency to gain electrons in a reduction reaction.
-
Catalysis: Many reactions involving SO₃ utilize catalysts to increase the reaction rate. The oxidation state of sulfur plays a role in catalyst selection and mechanism.
-
Environmental Chemistry: The release of SO₃ into the atmosphere contributes to acid rain, which highlights the environmental importance of understanding its chemical properties and reactivity. The +6 oxidation state's impact on atmospheric chemistry cannot be ignored.
Conclusion
Determining the oxidation number of sulfur in SO₃ involves applying fundamental rules of oxidation state assignment. The calculated +6 oxidation state is not merely a numerical value; it provides crucial insight into SO₃'s chemical properties, reactivity, and significance in various industrial and environmental processes. Understanding this oxidation state is vital for grasping the chemical behavior of this important compound and its role in a broader chemical context. The ability to accurately calculate and interpret oxidation numbers is a fundamental skill in chemistry, essential for both introductory and advanced studies. The information presented here provides a solid foundation for further exploration of sulfur chemistry and related topics.
Latest Posts
Latest Posts
-
Which Of The Following Is An Advantage Of Exporting
Apr 08, 2025
-
Identify The Statement Below That Is Incorrect
Apr 08, 2025
-
Which Statement Is True Regarding The Theory Of Natural Selection
Apr 08, 2025
-
State The Law Of Multiple Proportion
Apr 08, 2025
-
Ode To The West Wind Summary And Analysis
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of S In So3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.