All Elements In The Series Are Radioactive
News Leon
Mar 20, 2025 · 6 min read
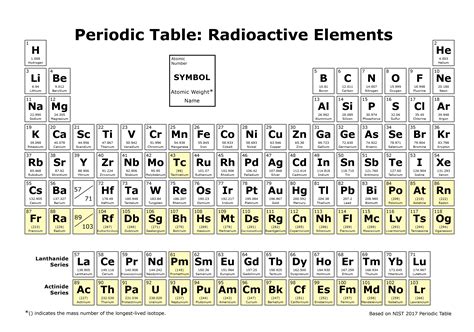
Table of Contents
All Elements in the Series Are Radioactive: Exploring the World of Radioactive Isotopes
The periodic table, a seemingly innocuous chart organizing the building blocks of matter, holds a fascinating secret: radioactivity isn't confined to a few exotic elements tucked away in obscure corners. While some elements are predominantly stable, many possess radioactive isotopes – versions of the element with unstable nuclei that decay over time, emitting radiation. This article delves into the world of radioactive isotopes, exploring why some elements are entirely radioactive, the types of radiation emitted, their detection, applications, and the crucial safety measures surrounding their handling.
Understanding Radioactivity and Isotopes
Before we delve into the specifics of entirely radioactive element series, let's establish a fundamental understanding of radioactivity and isotopes.
Isotopes: Variations on a Theme
Elements are defined by their atomic number—the number of protons in their nucleus. However, atoms of the same element can have varying numbers of neutrons. These variations are called isotopes. Some isotopes are stable, meaning their nuclei remain intact indefinitely. Others are unstable, or radioactive, meaning their nuclei spontaneously decay, transforming into different elements and emitting radiation in the process. This decay is governed by the element's half-life – the time it takes for half of a sample to decay. Half-lives can range from fractions of a second to billions of years.
Types of Radioactive Decay
Radioactive decay occurs through several mechanisms, each emitting different types of radiation:
-
Alpha Decay: This involves the emission of an alpha particle, consisting of two protons and two neutrons (essentially a helium nucleus). Alpha particles are relatively heavy and have low penetrating power, easily stopped by a sheet of paper or skin.
-
Beta Decay: This involves the emission of a beta particle, which can be an electron (beta-minus decay) or a positron (beta-plus decay). Beta particles are lighter than alpha particles and have greater penetrating power, requiring thicker materials like aluminum shielding.
-
Gamma Decay: This involves the emission of a gamma ray, a high-energy photon. Gamma rays are highly penetrating, requiring thick lead or concrete shielding.
-
Neutron Emission: Some radioactive isotopes emit neutrons, which are uncharged particles. Neutrons are highly penetrating and require specialized shielding.
Actinides: A Series Defined by Radioactivity
The actinide series, a row of elements at the bottom of the periodic table (atomic numbers 89-103), provides a prime example of a series where all elements are radioactive. These elements are characterized by their highly unstable nuclei, resulting in various decay modes and short to extremely long half-lives. Their radioactivity stems from the strong repulsive forces between the large number of protons in their nuclei, making them inherently unstable.
Notable Actinides and Their Characteristics
Let's examine some key actinides and their properties:
-
Actinium (Ac): All isotopes of actinium are radioactive. They undergo alpha and beta decay.
-
Thorium (Th): While some thorium isotopes have very long half-lives, making them seemingly stable over short timescales, all thorium isotopes are radioactive. They predominantly decay via alpha emission.
-
Protactinium (Pa): All isotopes of protactinium are radioactive, primarily undergoing alpha and beta decay.
-
Uranium (U): Uranium is famously radioactive, with its isotopes <sup>235</sup>U and <sup>238</sup>U being the most well-known. These undergo alpha decay, playing a crucial role in nuclear power and weaponry.
-
Neptunium (Np): All neptunium isotopes are radioactive, undergoing beta and alpha decay.
-
Plutonium (Pu): Plutonium is highly radioactive, known for its use in nuclear weapons. Its isotopes undergo alpha decay, along with spontaneous fission in some cases.
-
Americium (Am): Americium is known for its use in smoke detectors. All its isotopes are radioactive, undergoing alpha decay.
Beyond Actinides: Other Radioactive Series
While the actinides represent a complete series of radioactive elements, other elements exhibit significant radioactivity across their isotopic compositions.
Lanthanides: A Blend of Stability and Radioactivity
The lanthanides (atomic numbers 57-71), situated above the actinides, present a more complex picture. While many lanthanide isotopes are stable, several possess radioactive isotopes, though often with very long half-lives.
Transuranic Elements: Synthetic Radioactivity
The transuranic elements (atomic numbers greater than 92) are all synthetic, meaning they don't occur naturally in significant quantities. These are all radioactive, created through nuclear reactions. Their radioactivity stems from the extreme instability of their nuclei due to the large number of protons. Their half-lives vary widely, some decaying rapidly, others persisting for relatively longer periods.
Detecting and Measuring Radioactivity
Detecting and measuring radioactivity requires specialized instruments. Several common techniques exist:
-
Geiger counter: A Geiger counter detects ionizing radiation by measuring the ionization it causes in a gas-filled tube.
-
Scintillation detectors: These detectors use a scintillating material that emits light when radiation interacts with it. The light is then converted into an electrical signal.
-
Cloud chambers: Cloud chambers visualize the paths of ionizing radiation as condensation trails.
-
Nuclear emulsion: Nuclear emulsion is a photographic film sensitive to radiation, creating tracks that can be analyzed.
Applications of Radioactive Isotopes
Despite the inherent dangers, radioactive isotopes find numerous applications in various fields:
-
Medical Applications: Radioactive isotopes are used in medical imaging (PET scans, SPECT scans), radiotherapy, and in various diagnostic tests.
-
Industrial Applications: They are employed in gauging the thickness of materials, tracing the flow of fluids, and sterilizing medical equipment.
-
Scientific Research: Radioisotopes are crucial tools in various scientific fields, including archaeology, geology, and environmental science.
-
Nuclear Power: Uranium isotopes are the fuel for nuclear power plants, harnessing the energy released from nuclear fission.
Safety Precautions and Handling Radioactive Materials
Handling radioactive materials requires strict adherence to safety protocols to minimize exposure and prevent harmful effects. These include:
-
Shielding: Appropriate shielding materials (lead, concrete) must be used to reduce radiation exposure.
-
Distance: Maintaining a safe distance from radioactive sources significantly reduces exposure.
-
Time: Limiting the time spent near radioactive sources minimizes exposure.
-
Personal Protective Equipment (PPE): PPE, including lab coats, gloves, and respirators, should be worn when handling radioactive materials.
-
Monitoring: Regular monitoring of radiation levels using appropriate instruments is crucial.
-
Disposal: Safe and regulated disposal of radioactive waste is essential to prevent environmental contamination.
The Future of Radioactive Isotope Research
Research into radioactive isotopes continues to advance, with ongoing efforts to:
-
Develop new applications: Scientists are exploring new uses of radioactive isotopes in medicine, industry, and scientific research.
-
Improve safety protocols: Research focuses on improving the safety of handling and storing radioactive materials.
-
Develop more efficient detection methods: Ongoing efforts seek to develop more sensitive and efficient methods for detecting and measuring radiation.
-
Understanding the long-term effects of radiation: Research continues to investigate the long-term health effects of radiation exposure.
In conclusion, while many elements possess radioactive isotopes, the actinide series stands out as a unique collection where all isotopes of all elements are radioactive. Understanding the nature of radioactivity, the different types of decay, detection methods, safety precautions, and applications of radioactive isotopes is crucial for their responsible use and the safety of individuals and the environment. The continued study of these elements promises exciting advances in various scientific and technological fields.
Latest Posts
Latest Posts
-
What Is The Function Of The Stigma In A Flower
Mar 21, 2025
-
When A Tuning Fork Vibrates Over An Open Pipe
Mar 21, 2025
-
Is Supports Combustion A Physical Property
Mar 21, 2025
-
Give The Constituents Of Baking Powder
Mar 21, 2025
-
Which Of The Following Types Of Muscles Is Voluntary Muscle
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about All Elements In The Series Are Radioactive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
