According To The Kinetic Molecular Theory
News Leon
Mar 23, 2025 · 6 min read
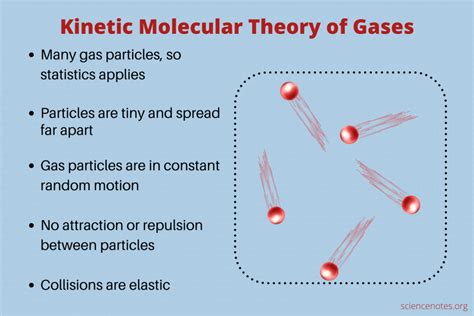
Table of Contents
According to the Kinetic Molecular Theory: A Deep Dive into the Behavior of Matter
The Kinetic Molecular Theory (KMT) is a cornerstone of chemistry, providing a powerful framework for understanding the behavior of matter at the macroscopic level based on the microscopic properties of its constituent particles. It explains properties like temperature, pressure, and volume, not as abstract concepts, but as direct consequences of the motion and interactions of atoms and molecules. This article will delve deep into the postulates of the KMT, exploring their implications and applications in various contexts.
The Postulates of the Kinetic Molecular Theory
The KMT rests upon several fundamental postulates, which, while simplified representations of reality, offer remarkably accurate predictions for the behavior of gases, and to a lesser extent, liquids and solids. These postulates are:
1. Matter is Composed of Tiny Particles in Constant, Random Motion
This seemingly simple statement is profound. It establishes that matter isn't a continuous entity, but rather a collection of discrete particles (atoms or molecules) constantly moving in unpredictable directions and speeds. This constant motion is the root of many macroscopic properties. The speed of these particles is directly related to the temperature of the system – higher temperature means faster motion.
2. The Volume of Individual Particles is Negligible Compared to the Total Volume of the Gas
This postulate is particularly relevant for gases. Gases are highly compressible because the space occupied by the gas particles themselves is insignificant compared to the vast empty space between them. In contrast, the volume of particles in liquids and solids is a significant fraction of the total volume, making them much less compressible. This explains why we can easily squeeze a balloon (gas) but not a rock (solid).
3. Attractive and Repulsive Forces Between Particles are Negligible
The KMT assumes that interactions between particles are minimal. This is a reasonable approximation for ideal gases, where the particles are far apart and the attractive forces are weak. However, real gases deviate from ideal behavior at high pressures and low temperatures because intermolecular forces become significant. These forces can cause particles to clump together, altering the predicted behavior.
4. Collisions Between Particles are Elastic
Elastic collisions mean that no kinetic energy is lost during the interaction. The total kinetic energy of the system remains constant before and after a collision. While real-world collisions aren't perfectly elastic, this assumption simplifies calculations and provides a good first-order approximation for many gas behaviors. The energy may transfer from one particle to another, changing their individual speeds, but the total energy remains constant.
5. The Average Kinetic Energy of Particles is Directly Proportional to Absolute Temperature
This is perhaps the most crucial postulate, linking the microscopic world (particle motion) to the macroscopic world (temperature). Absolute temperature (in Kelvin) is a direct measure of the average kinetic energy of the particles. At a given temperature, all gases have the same average kinetic energy, regardless of their mass. This means that lighter particles move faster than heavier particles at the same temperature.
Applications and Implications of the Kinetic Molecular Theory
The KMT isn't just a theoretical framework; it has practical applications across various fields. Understanding its implications allows us to explain and predict a wide range of phenomena.
1. Explaining Gas Laws
The KMT provides a microscopic explanation for the macroscopic gas laws, such as Boyle's Law, Charles's Law, and Avogadro's Law.
-
Boyle's Law (P₁V₁ = P₂V₂): At constant temperature, the volume of a gas is inversely proportional to its pressure. The KMT explains this by noting that increasing pressure forces the particles closer together, reducing the volume.
-
Charles's Law (V₁/T₁ = V₂/T₂): At constant pressure, the volume of a gas is directly proportional to its absolute temperature. As temperature increases, particles move faster, leading to more collisions and expansion, increasing the volume.
-
Avogadro's Law (V₁/n₁ = V₂/n₂): At constant temperature and pressure, the volume of a gas is directly proportional to the number of moles of gas. More moles mean more particles, leading to a larger volume.
2. Diffusion and Effusion
The KMT explains the processes of diffusion (the spreading of gas particles throughout a volume) and effusion (the escape of gas particles through a small hole). Lighter particles, possessing higher average speeds at a given temperature, diffuse and effuse faster than heavier particles. Graham's Law of Effusion is a direct consequence of this postulate.
3. Real Gases vs. Ideal Gases
The KMT provides a model for ideal gases, which perfectly follow the gas laws. However, real gases deviate from ideal behavior, especially at high pressures and low temperatures. This deviation arises from the limitations of the KMT's postulates: real gases have non-negligible particle volumes and experience intermolecular forces. The van der Waals equation is an example of a modified equation that accounts for these deviations.
4. Understanding States of Matter
While primarily developed for gases, the KMT concepts can be extended to liquids and solids. In liquids, particles are closer together and have stronger intermolecular forces, resulting in less freedom of movement compared to gases. In solids, particles are tightly packed and exhibit very limited movement, typically only vibrations around fixed positions. The differences in particle arrangement and movement explain the distinct properties of each state.
5. Predicting Reaction Rates
The KMT also plays a role in understanding chemical reaction rates. The rate of a reaction depends on the frequency and effectiveness of collisions between reactant particles. Higher temperatures lead to faster particle movement and more frequent, higher-energy collisions, increasing the reaction rate.
Limitations of the Kinetic Molecular Theory
While incredibly useful, the KMT has limitations:
-
Ideal Gas Assumption: The KMT assumes ideal gas behavior, which is only an approximation for real gases. Real gases deviate from ideal behavior, especially at high pressures and low temperatures, where intermolecular forces become significant.
-
Simplified Model: The KMT simplifies the complex interactions between particles. It ignores factors like the shape and size of molecules and the complexities of intermolecular forces. More sophisticated models are necessary for accurate predictions in certain situations.
-
Quantum Effects: The KMT doesn't account for quantum mechanical effects, which are significant for small particles or at very low temperatures.
Conclusion: A Powerful Framework for Understanding Matter
The Kinetic Molecular Theory, despite its limitations, remains a powerful and indispensable framework for understanding the behavior of matter. It bridges the gap between the macroscopic properties we observe and the microscopic motion of atoms and molecules. Its postulates provide a foundational understanding of gas laws, diffusion, effusion, and the differences between the states of matter. While not perfect, the KMT's simplicity and predictive power make it a cornerstone of chemical understanding, continually refined and extended to encompass a more complete and nuanced picture of the physical world. Further research continues to refine our understanding of intermolecular forces and the behavior of matter under extreme conditions, building upon the enduring legacy of the KMT. Its ability to provide a readily understandable explanation of complex phenomena makes it an essential tool for anyone seeking to comprehend the fundamental nature of matter. The ongoing advancements in computational chemistry and molecular dynamics simulations are further enhancing our ability to refine and extend the KMT's explanatory power, making it a continually evolving and essential part of the scientific landscape.
Latest Posts
Latest Posts
-
H2o2 H2o O2 What Type Of Reaction
Mar 24, 2025
-
List Of All Perfect Square Numbers
Mar 24, 2025
-
A Mixture Of Sand And Water Is A
Mar 24, 2025
-
Two More Than 4 Times A Number Is 18
Mar 24, 2025
-
What Is The Bond Order Of Co
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about According To The Kinetic Molecular Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
