A Tank Contains Water On Top Of Mercury
News Leon
Mar 16, 2025 · 5 min read
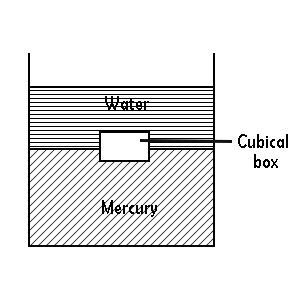
Table of Contents
A Tank Contains Water on Top of Mercury: Exploring the Physics and Applications
A seemingly simple scenario – a tank containing water on top of mercury – presents a fascinating exploration into fluid dynamics, density differences, and various scientific principles. This seemingly mundane setup reveals complexities relevant to diverse fields, from engineering and chemistry to environmental science. Let's delve into the intricacies of this system, examining its physical properties, practical applications, and potential challenges.
Understanding the Density Differential: Why Water Sits on Top
The fundamental principle governing this arrangement is density. Mercury, a liquid metal, boasts a significantly higher density (approximately 13.6 g/cm³) than water (approximately 1 g/cm³). This substantial difference is the reason why water, the less dense liquid, floats on top of the mercury. Imagine trying to submerge a cork in water; the same principle applies here, but on a larger scale. The denser mercury settles to the bottom, forming a stable base layer upon which the water rests.
The Role of Gravity: A Constant Force
Gravity plays a crucial role in maintaining this layered structure. The gravitational force acts on both liquids, pulling them downwards. However, the greater density of mercury means it experiences a stronger gravitational pull per unit volume, leading to its settling at the bottom. This gravitational force, coupled with the inherent density difference, ensures the stability of the water-mercury interface.
Pressure Distribution within the Tank: A Gradual Increase
The pressure within the tank isn't uniform; it increases with depth. This is a direct consequence of the weight of the overlying fluids. At any point within the water layer, the pressure is the sum of the hydrostatic pressure due to the water column above that point and the hydrostatic pressure due to the mercury column below the water layer. The pressure exerted on the bottom of the tank is significantly higher than the pressure at the water-mercury interface or at the surface of the water.
Calculating Pressure at Different Depths
The pressure (P) at a specific depth (h) can be calculated using the following formula:
P = ρgh
where:
- ρ (rho) represents the density of the fluid
- g represents the acceleration due to gravity
- h represents the depth
To determine the total pressure at the bottom of the tank, one must calculate the pressure due to the water column and add it to the pressure due to the mercury column, using the appropriate density and height for each liquid.
Interfacial Tension: The Boundary Between Two Worlds
The boundary between the water and mercury layers isn't a perfectly sharp line; interfacial tension plays a significant role. Interfacial tension is the force that exists at the interface between two immiscible liquids. In this case, the water and mercury molecules interact differently, creating a surface tension that tries to minimize the surface area between them. This tension influences the shape and stability of the interface, especially at smaller scales.
Minimizing the Interface: A Natural Phenomenon
The system naturally seeks to minimize the interfacial area, resulting in a relatively flat interface between the water and mercury layers under normal conditions. However, factors such as external forces or disturbances can temporarily disrupt this flat interface, creating ripples or fluctuations.
Practical Applications and Real-World Scenarios
While seemingly simple, this water-on-mercury system has numerous real-world applications and relevance to various scientific and engineering scenarios:
1. Mercury Barometers and Manometers
Historically, mercury barometers have been used to measure atmospheric pressure. The height of the mercury column in a barometer directly relates to the atmospheric pressure. While less common today due to mercury's toxicity, the principle remains relevant in understanding pressure measurement. Similarly, manometers employing mercury can be used to precisely measure pressure differences in various systems.
2. Density Measurement and Calibration
The distinct density difference between water and mercury allows for precise density measurements and calibration of measuring instruments. By observing the behavior of objects within this two-layered system, one can infer their density relative to water and mercury.
3. Chemical Engineering and Separations
In certain chemical processes, the density difference between liquids might be exploited for separation purposes. While not directly utilizing a water-mercury system, the principle of density-based separation is relevant and is employed in various industrial processes.
4. Environmental Science and Mercury Contamination
Understanding the behavior of mercury in different environments, including its interaction with water, is crucial in environmental science. Studies involving mercury contamination often consider its behavior in aquatic systems, highlighting the importance of density and fluid dynamics in environmental modeling and remediation.
Potential Challenges and Safety Precautions
Working with mercury presents significant safety challenges. Mercury is highly toxic, and its vapor can pose serious health risks. Therefore, any application or experiment involving mercury requires stringent safety measures:
- Proper ventilation: Adequate ventilation is essential to minimize exposure to mercury vapor.
- Protective equipment: Protective gear, including gloves, eye protection, and respirators, must be worn.
- Spill containment: Detailed spill response plans should be in place to mitigate the risks associated with accidental mercury spills.
- Disposal procedures: The disposal of mercury and mercury-contaminated materials must adhere to strict regulations to protect the environment.
Further Exploration: Advanced Concepts
The water-on-mercury system offers opportunities for exploring more complex concepts:
- Fluid dynamics simulations: Computational fluid dynamics (CFD) can be used to model the behavior of the two liquids under various conditions, such as mixing, stirring, or the introduction of other substances.
- Interface stability and perturbation: Investigating the stability of the interface under different conditions, such as vibration or external forces, is an area of ongoing research.
- The impact of temperature: Temperature variations affect the densities of both water and mercury, leading to changes in the pressure distribution and the stability of the system.
Conclusion: A Simple System with Profound Implications
The seemingly straightforward system of a tank containing water on top of mercury provides a rich platform for studying fundamental physical principles, with implications spanning diverse scientific and engineering fields. While the simplicity of the setup is appealing, it's crucial to recognize the inherent dangers of working with mercury and to take appropriate precautions. By understanding the interplay of density, pressure, and interfacial tension, we can gain valuable insights into the behavior of liquids and their application in various contexts. The seemingly simple act of layering two liquids reveals a world of fascinating physics and practical applications, demonstrating the power of careful observation and scientific investigation.
Latest Posts
Latest Posts
-
Which Bond Is The Most Polar
Mar 17, 2025
-
Burning Of Candle Is Chemical Change
Mar 17, 2025
-
An Earth Satellite Moves In A Circular Orbit
Mar 17, 2025
-
What Is The Value Of K In Physics
Mar 17, 2025
-
The Study Of Tissues With A Microscope Is Called
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about A Tank Contains Water On Top Of Mercury . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
