A Lithium Atom Has Three Electrons
News Leon
Mar 19, 2025 · 6 min read
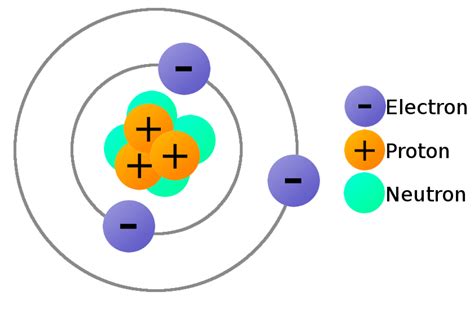
Table of Contents
A Lithium Atom Has Three Electrons: Delving into the Fundamentals of Atomic Structure
The seemingly simple statement, "a lithium atom has three electrons," opens a door to a fascinating world of atomic structure, chemical behavior, and the fundamental principles governing the universe. While the number itself might seem insignificant, it's the foundation upon which lithium's unique properties and its crucial role in various applications are built. This article will explore this seemingly simple fact in detail, unraveling the intricacies of electron configuration, the implications for chemical bonding, and the significance of lithium's unique atomic structure in the context of its widespread uses.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the specifics of lithium's three electrons, let's establish a basic understanding of atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element; all lithium atoms have three protons.
- Neutrons: Neutrally charged particles also found in the nucleus. The number of neutrons can vary within an element, leading to isotopes (e.g., Lithium-6 and Lithium-7).
- Electrons: Negatively charged particles orbiting the nucleus in distinct energy levels or shells. These electrons are responsible for chemical bonding and determine an element's chemical properties. The number of electrons in a neutral atom equals the number of protons.
Lithium's Three Electrons: Configuration and Significance
The crucial point about lithium is that it possesses three electrons. This seemingly small number has profound consequences for its chemical behavior and its place in the periodic table. These three electrons are distributed across energy levels according to the Aufbau principle and Hund's rule.
Electron Configuration: 1s²2s¹
Lithium's electron configuration is written as 1s²2s¹. This notation signifies:
- 1s²: Two electrons occupy the lowest energy level (n=1), specifically the s subshell. The s subshell can hold a maximum of two electrons.
- 2s¹: One electron occupies the next higher energy level (n=2), also in the s subshell.
This configuration is pivotal because it dictates lithium's reactivity. The outermost electron, residing in the 2s orbital, is relatively loosely bound to the nucleus. This valence electron is readily available for participation in chemical bonding, making lithium highly reactive.
Implications for Chemical Bonding: Why Lithium is Reactive
The presence of a single valence electron makes lithium highly reactive. It readily loses this electron to achieve a stable electron configuration, resembling the noble gas helium (which has a filled 1s orbital). This process is known as ionization, resulting in the formation of a Li⁺ ion. Lithium's strong tendency to lose an electron contributes to its:
- Low ionization energy: The energy required to remove the valence electron is relatively low.
- Electropositive nature: It readily loses electrons, behaving as a strong reducing agent.
- Formation of ionic compounds: It readily forms ionic bonds with electronegative elements like halogens (e.g., chlorine, fluorine) and oxygen.
Lithium's Unique Properties: A Consequence of Three Electrons
Lithium's unique properties, which make it indispensable in various applications, are all directly linked to its atomic structure and the presence of its three electrons:
-
Low density: Lithium is the lightest metal, possessing significantly lower density than other alkali metals. This low density is attributable to its atomic structure and the relatively large distance between the nucleus and its outermost electron.
-
High reactivity: As discussed earlier, the presence of a single valence electron makes lithium highly reactive, leading to its use in various chemical reactions and battery applications.
-
Electrochemical properties: The ease with which lithium loses its valence electron makes it an excellent choice for batteries. Lithium-ion batteries, widely used in portable electronics, electric vehicles, and energy storage systems, rely on lithium's electrochemical properties. The movement of lithium ions between the anode and cathode generates the electrical current.
-
Thermal conductivity: Lithium possesses high thermal conductivity, making it useful in heat transfer applications.
Lithium in Various Applications: From Batteries to Medicine
The unique properties stemming from its three electrons make lithium indispensable across a multitude of applications:
1. Lithium-ion Batteries: Powering Our Modern World
Lithium-ion batteries dominate the portable electronics market and are increasingly vital in electric vehicles and grid-scale energy storage. Their high energy density, long lifespan, and relatively low self-discharge rate make them superior to other battery technologies. The fundamental principle behind these batteries is the movement of lithium ions between the anode and cathode, a process directly enabled by lithium's atomic structure and its willingness to lose its valence electron.
2. Lubricants and Greases: Enhancing Performance
Lithium-based greases are widely used as lubricants due to their excellent high-temperature stability and water resistance. The lithium atoms interact with the grease components to form a stable lubricating film, providing superior performance in various applications.
3. Metallurgy: Improving Alloy Properties
Lithium is added to various alloys to enhance their properties, such as increasing strength and reducing weight. For example, lithium is used in aluminum alloys for aerospace applications.
4. Glass and Ceramics: Enhancing Durability
Lithium compounds are used in the production of glass and ceramics to improve their durability, thermal shock resistance, and other properties. The presence of lithium ions alters the crystal structure and strengthens the material.
5. Nuclear Reactions: A Unique Role in Energy Production
Lithium isotopes, particularly Lithium-6, play a role in nuclear fusion reactions, which is a potential source of clean energy. The interaction of lithium nuclei with neutrons in a fusion reactor produces tritium, another important fuel for fusion reactions.
6. Medicine and Therapeutics: Addressing Mental Health Challenges
Lithium salts are used in the treatment of certain mental illnesses, particularly bipolar disorder. While the exact mechanism of action is not fully understood, lithium's interaction with cellular processes is believed to stabilize mood and reduce the severity of manic and depressive episodes.
Isotopes of Lithium: Variations in Neutron Count
While the number of protons and electrons defines lithium as an element, the number of neutrons can vary. This leads to the existence of isotopes, which are atoms of the same element with different numbers of neutrons. The two most common isotopes are:
- Lithium-6 (⁶Li): Contains three protons and three neutrons.
- Lithium-7 (⁷Li): Contains three protons and four neutrons.
These isotopes have slightly different properties, particularly in terms of nuclear behavior. Lithium-6, for example, is used in nuclear applications due to its interaction with neutrons. However, the fundamental chemical properties dictated by the three electrons remain largely the same across both isotopes.
Conclusion: The Importance of Understanding Atomic Structure
The seemingly simple fact that a lithium atom has three electrons is a gateway to understanding a wide array of complex phenomena. From the fundamentals of atomic structure and chemical bonding to the development of advanced technologies and medical treatments, lithium's unique properties, all stemming from its three electrons, make it an element of immense importance in our modern world. Further research into lithium's behavior and its interactions at the atomic level continues to unveil new applications and further solidify its significance in various scientific and technological fields. The continuing exploration of this relatively simple element underscores the profound impact of fundamental scientific principles on our daily lives and the potential for future innovation.
Latest Posts
Latest Posts
-
Freezing Point Of Water On Celsius Scale
Mar 20, 2025
-
Graphite Is A Good Conductor Of Electricity
Mar 20, 2025
-
What Is 3 4 Pound In Ounces
Mar 20, 2025
-
Crossing Over Occurs During Which Stage Of Meiosis
Mar 20, 2025
-
Is 48 A Multiple Of 8
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about A Lithium Atom Has Three Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
