Graphite Is A Good Conductor Of Electricity
News Leon
Mar 20, 2025 · 5 min read
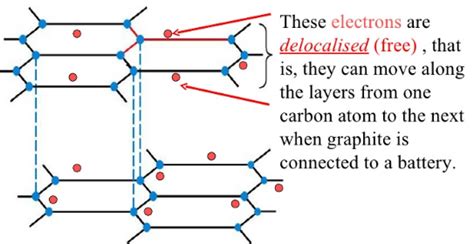
Table of Contents
Graphite: A Surprisingly Good Conductor of Electricity
Graphite, a form of carbon, is often overlooked in discussions of electrical conductivity. More frequently, we hear about metals like copper and silver. However, graphite possesses a unique atomic structure that makes it a surprisingly good conductor of electricity, though not as good as copper. This property, alongside its other remarkable characteristics, makes it a crucial material in various technological applications. This article will delve deep into the reasons behind graphite's electrical conductivity, exploring its atomic structure, applications, and comparisons with other conductors.
Understanding Graphite's Atomic Structure: The Key to Conductivity
The secret to graphite's electrical conductivity lies in its unique atomic arrangement. Unlike diamond, another allotrope of carbon, graphite's carbon atoms are arranged in a layered structure. Each layer consists of a hexagonal lattice of carbon atoms, strongly bonded together through covalent bonds within the plane. These strong bonds are responsible for graphite's strength and hardness within the layers.
The Weak Interlayer Forces: Enabling Electron Mobility
However, what truly distinguishes graphite is the weak van der Waals forces that hold these layers together. This weak bonding between layers allows them to easily slide past each other, contributing to graphite's characteristic softness and lubricating properties. More importantly for electrical conductivity, this weak interlayer bonding allows for significant electron mobility.
Delocalized Electrons: The Charge Carriers
The crucial aspect of graphite's atomic structure related to its conductivity is the presence of delocalized electrons. Each carbon atom in the hexagonal lattice contributes one electron to a delocalized pi (π) electron system. These electrons aren't bound to any single atom; instead, they are free to move throughout the entire layer. This sea of delocalized electrons is responsible for graphite's ability to conduct electricity.
The Mechanism of Electrical Conductivity in Graphite
When an electric field is applied across a graphite sample, these delocalized electrons are readily accelerated, creating an electric current. The ease with which these electrons move dictates the material's conductivity. The higher the mobility of these charge carriers, the higher the conductivity.
Anisotropy of Conductivity: A Directional Dependence
It's important to note that graphite's conductivity isn't uniform in all directions. Due to the layered structure, conductivity is significantly higher within the layers (in-plane conductivity) than between the layers (interlayer conductivity). This property is called anisotropy. The strong covalent bonds within the layers provide a path of least resistance for electron flow, while the weak van der Waals forces between layers hinder electron movement perpendicular to the layers.
Comparing Graphite's Conductivity to Other Materials
While graphite is a good conductor, it's not the best. Metals like copper and silver exhibit far superior electrical conductivity. This difference stems from the nature of their electron structure and bonding.
Metals: A Sea of Free Electrons
Metals possess a "sea" of free electrons that are not bound to specific atoms. This allows for extremely high electron mobility, resulting in excellent conductivity. Copper, in particular, is widely used in electrical wiring due to its high conductivity and affordability.
Semiconductors: Intermediate Conductivity
Semiconductors like silicon and germanium have intermediate conductivity. Their conductivity can be manipulated by doping, the introduction of impurities that alter the number of charge carriers. This property makes them crucial in electronic devices.
Insulators: Negligible Conductivity
In contrast, insulators like rubber and glass have negligible conductivity. Their electrons are tightly bound to their atoms, preventing significant electron movement.
Factors Affecting Graphite's Electrical Conductivity
Several factors influence the electrical conductivity of graphite:
Purity: The Impact of Impurities
The purity of the graphite significantly impacts its conductivity. Impurities can act as scattering centers, disrupting the flow of electrons and reducing conductivity. Higher purity graphite generally exhibits higher conductivity.
Temperature: The Temperature Dependence
Temperature also affects conductivity. At higher temperatures, lattice vibrations increase, leading to increased scattering of electrons and reduced conductivity. Conversely, at lower temperatures, conductivity generally increases.
Pressure: The Effect of Compression
Applying pressure to graphite can alter its conductivity. Compressing the layers reduces the distance between them, potentially increasing interlayer conductivity, although the effect is complex and depends on the pressure magnitude.
Orientation: The Importance of Alignment
The orientation of the graphite layers relative to the direction of current flow significantly impacts conductivity. As mentioned earlier, in-plane conductivity is significantly higher than interlayer conductivity. This directional dependence needs to be considered in applications.
Applications Leveraging Graphite's Conductivity
The unique conductivity properties of graphite, alongside its other characteristics, have led to its extensive use in various applications:
Batteries: Anode Material
Graphite is a crucial component in lithium-ion batteries, serving as the anode material. Its ability to intercalate lithium ions and its conductivity are essential for battery performance.
Electrodes: In Various Electrochemical Processes
Graphite is utilized as an electrode material in various electrochemical processes, including electrolysis and fuel cells, where its conductivity facilitates electron transfer.
Electronic Components: Resistors and Conductors
Graphite's conductivity makes it suitable for use in some electronic components, including resistors and conductors, although its conductivity is typically lower than metals commonly used in electronics.
Lubricants: Reducing Friction
Graphite's lubricating properties, arising from the weak interlayer forces, often make it part of a conductive lubricant mix. These are used in high-temperature or high-pressure applications where standard lubricants may fail.
Pencil "Lead": Writing and Drawing
Even the humble pencil "lead" leverages graphite's conductivity (though indirectly) – the material allows the transfer of graphite onto paper, making writing possible.
Conclusion: Graphite – A Versatile Conductive Material
Graphite stands out as a unique material with surprising electrical conductivity. Its layered structure, with its delocalized electrons and weak interlayer forces, provides a unique balance of properties. While not as conductive as copper or silver, graphite's conductivity, combined with its other attributes such as its lubricating qualities and thermal stability, makes it indispensable in a wide array of technological applications. Understanding the intricate relationship between its atomic structure and its conductivity allows us to fully appreciate its versatility and importance in modern technology. Further research into controlling and enhancing its conductivity could unlock even more exciting applications in the future.
Latest Posts
Latest Posts
-
A Complete Virus Particle Is Called A
Mar 20, 2025
-
Which Of The Following Is Not A Protein Function
Mar 20, 2025
-
Explain How Ionic Compounds Dissolve In Water
Mar 20, 2025
-
The Additional Satisfaction Of Consuming A Good Or Service Is
Mar 20, 2025
-
Which Of The Following Organisms Has No Specialized Respiratory Structures
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Graphite Is A Good Conductor Of Electricity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
