A Gas Expands From I To F In The Figure
News Leon
Mar 24, 2025 · 6 min read
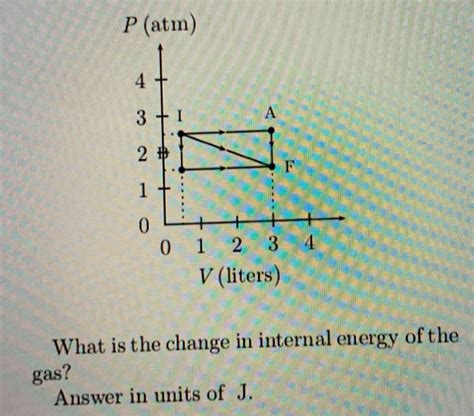
Table of Contents
A Gas Expands from I to F: Exploring Thermodynamic Processes
Understanding how gases behave under varying conditions is fundamental to numerous scientific and engineering disciplines. This article delves into the thermodynamic process represented by a gas expanding from point I to point F on a pressure-volume (PV) diagram. We will explore various aspects, including the types of processes involved, the calculation of work done, heat transfer, and the overall change in internal energy. We'll also touch upon the implications for different thermodynamic systems and the applications of this fundamental principle.
Understanding the Pressure-Volume Diagram
Before we delve into the specifics of the expansion from I to F, it's crucial to understand the significance of the pressure-volume (PV) diagram. This graphical representation plots pressure (P) on the y-axis and volume (V) on the x-axis. Each point on the diagram represents a specific state of the gas, defined by its pressure and volume. A process, such as expansion or compression, is represented by a curve connecting different states. The shape of this curve reveals important information about the nature of the process.
Key Features of the PV Diagram
- Isobaric Process: A horizontal line on a PV diagram signifies a constant pressure process. The pressure remains unchanged while the volume changes.
- Isochoric Process: A vertical line represents a constant volume process. The volume remains constant while the pressure changes.
- Isothermal Process: A curve that follows the equation PV = constant signifies a constant temperature process.
- Adiabatic Process: A steeper curve than an isothermal process indicates an adiabatic process where no heat exchange occurs with the surroundings.
Analyzing the Expansion from I to F
Let's assume, for the sake of this discussion, that the expansion from point I to point F involves a combination of processes, potentially including isobaric, isochoric, isothermal, or adiabatic segments. The exact nature of the process will depend on the specific conditions under which the expansion occurs, such as the presence or absence of heat transfer and the constraints imposed on the system.
Scenario 1: A Multi-Step Expansion (Isobaric and Isochoric)
One possibility is that the gas first expands isobarically from I to an intermediate point, let's call it J, at constant pressure. Then, it expands isochorically from J to F at constant volume.
-
Step 1: Isobaric Expansion (I to J): During this stage, the pressure remains constant, and the volume increases. The work done by the gas is calculated as:
W = PΔV, where P is the constant pressure and ΔV is the change in volume (V<sub>J</sub> - V<sub>I</sub>). The heat transferred, Q, during this step depends on the specific heat capacity of the gas at constant pressure and the temperature change. -
Step 2: Isochoric Expansion (J to F): In this step, the volume remains constant, and the pressure decreases. No work is done by the gas during an isochoric process because
W = PΔV = P(0) = 0. However, heat transfer, Q, might still occur, leading to a change in the internal energy of the gas.
The total work done during this multi-step expansion is simply the sum of the work done in each individual step. The total heat transfer is also the sum of the heat transfers in each step. The overall change in internal energy (ΔU) can be determined using the First Law of Thermodynamics: ΔU = Q - W.
Scenario 2: An Isothermal Expansion (I to F)
Another possibility is that the expansion from I to F occurs isothermally, meaning the temperature remains constant throughout the process. In this case, the relationship between pressure and volume follows the ideal gas law: PV = nRT, where n is the number of moles of gas, R is the ideal gas constant, and T is the absolute temperature.
The work done during an isothermal expansion is given by: W = nRT ln(V<sub>F</sub>/V<sub>I</sub>). Since the temperature is constant, any heat added to the system is entirely used to do work. Thus, Q = W, and the change in internal energy (ΔU) is zero because the internal energy of an ideal gas depends only on temperature.
Scenario 3: An Adiabatic Expansion (I to F)
In an adiabatic expansion, no heat exchange occurs between the gas and its surroundings (Q=0). The relationship between pressure and volume for an adiabatic process is given by: PV<sup>γ</sup> = constant, where γ is the adiabatic index (ratio of specific heats, C<sub>p</sub>/C<sub>v</sub>).
The work done during an adiabatic expansion is more complex to calculate and typically involves integration. The change in internal energy is equal to the negative of the work done (ΔU = -W) because no heat is exchanged.
Implications for Different Thermodynamic Systems
The behavior of a gas expanding from I to F varies depending on the type of thermodynamic system involved.
-
Closed System: In a closed system, the mass of the gas remains constant during the expansion. The processes described above apply directly to a closed system.
-
Open System: In an open system, mass can flow in or out of the system. The analysis becomes more complex because the mass of the gas is not constant. The work done and heat transfer need to account for the changes in mass.
-
Isolated System: In an isolated system, neither mass nor energy can cross the system boundaries. Therefore, the expansion from I to F would not be possible in a truly isolated system without external intervention.
Applications and Real-World Examples
The principles governing gas expansion have wide-ranging applications in various fields.
-
Internal Combustion Engines: The expansion of gases in the cylinders of internal combustion engines is a prime example of this principle. The controlled expansion of hot gases generated from combustion drives the pistons, producing mechanical work.
-
Refrigeration and Air Conditioning: The expansion of refrigerants in refrigeration cycles is crucial for cooling. The expansion leads to a decrease in temperature, enabling heat absorption from the surroundings.
-
Power Generation: Many power generation systems, including steam turbines, rely on the expansion of gases (steam in this case) to generate mechanical energy.
-
Aerospace Engineering: Understanding gas expansion is critical in designing rockets and jet engines. The expansion of propellant gases provides the thrust needed for propulsion.
Conclusion
The expansion of a gas from point I to point F on a PV diagram presents a rich area of study in thermodynamics. The specific behavior of the gas depends heavily on the conditions of the expansion, including whether it occurs at constant pressure, constant volume, constant temperature, or adiabatically. By analyzing the work done, heat transfer, and the overall change in internal energy, we can gain a deeper understanding of the thermodynamic principles governing this fundamental process and appreciate its far-reaching applications in diverse technological fields. Further exploration could involve specific examples with numerical values, exploring the concept of reversible and irreversible processes, and delving into the microscopic behavior of gas molecules during expansion. This foundation provides a robust understanding for more advanced topics in thermodynamics and its applications.
Latest Posts
Latest Posts
-
How To Calculate Long Run Equilibrium Price
Mar 26, 2025
-
Draw The Ozonolysis Products Of 2 Methyl 2 Pentene
Mar 26, 2025
-
The Correct Name For Feo Is
Mar 26, 2025
-
What Is The Distance Between Points A And B
Mar 26, 2025
-
What Does The Kinetic Theory Of Matter State
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about A Gas Expands From I To F In The Figure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
