What Does The Kinetic Theory Of Matter State
News Leon
Mar 26, 2025 · 6 min read
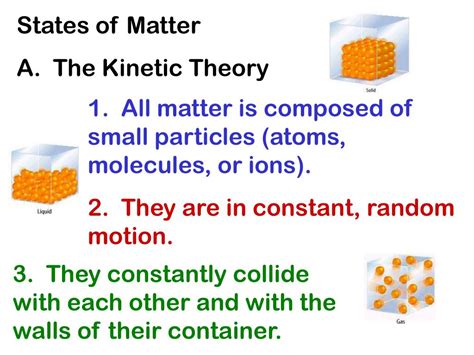
Table of Contents
What Does the Kinetic Theory of Matter State? A Deep Dive
The kinetic theory of matter is a fundamental concept in physics that explains the macroscopic properties of matter—like temperature, pressure, and volume—based on the microscopic behavior of its constituent particles (atoms and molecules). It's a powerful model that bridges the gap between the observable world and the unseen world of atoms and molecules, providing a framework for understanding a wide range of phenomena. This article will delve deep into the postulates of the kinetic theory, its applications, limitations, and its significance in various scientific fields.
The Core Postulates of the Kinetic Theory of Matter
At the heart of the kinetic theory lie several key postulates that collectively describe the behavior of matter at the atomic and molecular level. These postulates are:
1. Matter is Composed of Tiny Particles:
This seems obvious today, but it was a revolutionary idea when the kinetic theory was first developed. The theory posits that all matter, regardless of its state (solid, liquid, or gas), is made up of incredibly small particles—atoms or molecules—that are constantly in motion. The nature of this motion varies depending on the state of matter.
2. These Particles are in Constant, Random Motion:
This is where the "kinetic" part of the theory comes in. "Kinetic" refers to motion, and the theory emphasizes that these particles are never truly at rest. They are constantly jiggling, vibrating, rotating, and translating (moving from one place to another). The speed and type of motion depend on factors like temperature and the nature of the particles. This random motion is crucial for understanding many physical properties.
3. Particles Collide with Each Other and with the Walls of Their Container:
These collisions are elastic, meaning that kinetic energy is conserved during the collision. In other words, no energy is lost during the collision. These collisions are responsible for the pressure exerted by a gas, as the particles bombard the walls of their container, transferring momentum.
4. The Forces of Attraction Between Particles are Negligible (for Ideal Gases):
This postulate is especially important when considering ideal gases. An ideal gas is a theoretical construct where the attractive forces between particles are completely ignored. While no real gas is perfectly ideal, many gases behave approximately ideally under certain conditions (low pressure and high temperature). In real gases, intermolecular forces do play a significant role and deviate from ideal gas behavior.
5. The Average Kinetic Energy of the Particles is Directly Proportional to the Absolute Temperature:
This is a fundamental connection between the microscopic world and the macroscopic world. Temperature is a measure of the average kinetic energy of the particles in a substance. As temperature increases, the average kinetic energy of the particles increases, resulting in faster motion. This relationship is crucial for understanding thermal phenomena.
Applying the Kinetic Theory: Understanding Different States of Matter
The kinetic theory helps explain the differences between the three common states of matter—solid, liquid, and gas—based on the strength of the intermolecular forces and the degree of particle motion:
Solids:
In solids, the particles are tightly packed together and held in relatively fixed positions by strong intermolecular forces. The particles vibrate in place but do not have enough kinetic energy to overcome these forces and move freely. This explains their rigid structure and fixed volume and shape.
Liquids:
Liquids have weaker intermolecular forces than solids, allowing the particles to move more freely. The particles are still close together, but they can slide past one another, explaining the liquid's ability to flow and take the shape of its container. The volume of a liquid remains relatively constant.
Gases:
Gases have very weak intermolecular forces, and the particles are far apart and move rapidly and randomly in all directions. They collide frequently with each other and the walls of their container. This explains the gas's ability to expand to fill any container and its compressibility.
Beyond the Basics: Real Gases and Deviations from Ideal Behavior
While the ideal gas model is useful for many applications, real gases deviate from ideal behavior, especially at high pressures and low temperatures. This deviation stems from the fact that real gases do experience intermolecular forces. These forces can cause:
-
Reduced pressure: Attractive forces between gas molecules pull them slightly closer together, reducing the number of collisions with the container walls and thus lowering the observed pressure.
-
Increased volume: At lower temperatures, the attractive forces can become significant enough to cause the gas molecules to clump together, taking up less volume than predicted by the ideal gas law.
The van der Waals equation is a modified version of the ideal gas law that attempts to account for these intermolecular forces and provide a more accurate description of real gases. It introduces correction factors to account for the finite size of the gas molecules and the intermolecular attractive forces.
Applications of the Kinetic Theory
The kinetic theory's impact extends far beyond the simple explanation of states of matter. Its principles are crucial in understanding and modeling numerous phenomena, including:
-
Diffusion and Effusion: The random motion of particles is central to understanding how gases mix (diffusion) and how they escape through tiny holes (effusion). Graham's Law of Effusion is a direct consequence of the kinetic theory.
-
Brownian Motion: The erratic, random movement of microscopic particles suspended in a fluid (like pollen grains in water), observed by Robert Brown, is explained by the constant bombardment of the particles by the surrounding fluid molecules, a direct consequence of their kinetic energy.
-
Osmosis: The movement of solvent molecules across a semipermeable membrane from a region of high concentration to a region of low concentration can be understood using the kinetic theory, relating to the different kinetic energies of the particles on either side of the membrane.
Limitations of the Kinetic Theory
Despite its successes, the kinetic theory has limitations:
-
It's a simplified model: The kinetic theory simplifies the complexity of real interactions at the atomic and molecular level. It doesn't account for all the intricacies of particle interactions, such as quantum effects or detailed shapes of molecules.
-
It struggles with condensed phases: While the kinetic theory provides a reasonable explanation for gases, its application to liquids and solids is more complex and less accurate due to the stronger intermolecular forces and more intricate particle interactions. More sophisticated models are needed to fully understand the behavior of condensed matter.
-
It assumes perfectly elastic collisions: While a useful approximation, real collisions are not perfectly elastic. Some kinetic energy is lost as vibrational energy or heat during collisions.
Conclusion: The Enduring Significance of the Kinetic Theory
The kinetic theory of matter remains a cornerstone of modern physics and chemistry. While it's a simplified model, it provides a remarkably successful framework for understanding the macroscopic properties of matter based on the microscopic behavior of its constituent particles. Its applications are vast and far-reaching, impacting diverse fields from materials science to atmospheric science. Its limitations should be understood, prompting continued refinement and development of more sophisticated models, but its core principles continue to illuminate our understanding of the physical world. Future research in nanotechnology and materials science will continue to benefit from the foundation laid by this fundamental theory.
Latest Posts
Latest Posts
-
What Is The Difference Between A Democracy And Dictatorship
Mar 29, 2025
-
Bill Is To Law As Larva Is To
Mar 29, 2025
-
Which Organelle Is Responsible For Cellular Respiration
Mar 29, 2025
-
What Is The Ph For A Neutral Solution
Mar 29, 2025
-
Is Black Hair A Dominant Trait
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Does The Kinetic Theory Of Matter State . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
