What Is The Ph For A Neutral Solution
News Leon
Mar 29, 2025 · 6 min read
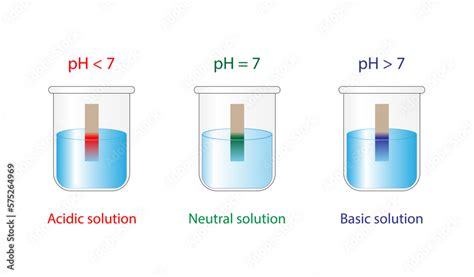
Table of Contents
What is the pH for a Neutral Solution? A Deep Dive into pH, Acidity, and Alkalinity
The question, "What is the pH for a neutral solution?" seems simple enough. The answer, however, opens the door to a fascinating world of chemistry, encompassing concepts crucial to various fields, from environmental science and medicine to agriculture and industrial processes. This comprehensive article will delve deep into the meaning of pH, explore what constitutes a neutral solution, and examine the factors that can influence pH measurements. We'll also discuss the importance of pH in different contexts and the methods used to measure and control it.
Understanding pH: The Power of Hydrogen
pH, a term derived from the phrase "potential of hydrogen," is a logarithmic scale that measures the acidity or alkalinity of an aqueous solution. It reflects the concentration of hydrogen ions (H⁺) present in the solution. The scale ranges from 0 to 14, with:
- pH 0-7: Acidic solutions. The lower the pH, the higher the concentration of H⁺ ions.
- pH 7: Neutral solutions. The concentration of H⁺ ions is equal to the concentration of hydroxide ions (OH⁻).
- pH 7-14: Alkaline (or basic) solutions. The higher the pH, the lower the concentration of H⁺ ions and the higher the concentration of OH⁻ ions.
The Logarithmic Nature of the pH Scale
It's crucial to understand that the pH scale is logarithmic, meaning that each whole number change represents a tenfold change in the concentration of H⁺ ions. For instance, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5. This logarithmic nature highlights the significant impact even small changes in pH can have.
Defining a Neutral Solution: pH 7 and the Water Equilibrium
A neutral solution is defined as a solution where the concentration of hydrogen ions (H⁺) is equal to the concentration of hydroxide ions (OH⁻). At 25°C (77°F), pure water exhibits this equilibrium, resulting in a pH of 7. This equilibrium is represented by the following equation:
2H₂O ⇌ H₃O⁺ + OH⁻
This equation shows that water molecules (H₂O) can self-ionize to form hydronium ions (H₃O⁺, often simplified to H⁺) and hydroxide ions (OH⁻). In pure water, the concentration of both ions is 1 x 10⁻⁷ moles per liter, leading to a pH of 7.
The Influence of Temperature on pH
It's important to note that the pH of a neutral solution is temperature-dependent. At temperatures above 25°C, the self-ionization of water increases, leading to a slightly higher concentration of both H⁺ and OH⁻ ions. Consequently, the pH of neutral water at higher temperatures will be slightly less than 7. Conversely, at temperatures below 25°C, the pH of neutral water will be slightly greater than 7. While the deviation is typically small, it's a factor to consider for precise measurements, particularly in scientific research.
Factors Affecting pH Measurements
Several factors can influence pH measurements and their accuracy:
- Temperature: As discussed, temperature significantly affects the self-ionization of water and, consequently, the pH of neutral solutions.
- Dissolved substances: The presence of dissolved salts, acids, or bases can drastically alter the pH of a solution. Even trace amounts of impurities can affect the accuracy of measurements.
- Electrode calibration: pH meters require regular calibration using standard buffer solutions to ensure accurate readings. Improper calibration can lead to significant errors.
- Electrode maintenance: Proper cleaning and storage of the pH electrode are vital for maintaining its accuracy and longevity. Contamination or damage to the electrode can affect readings.
- Sample preparation: The method of sample preparation can also influence pH measurements. For instance, improper mixing or aeration can affect the concentration of ions and, subsequently, the pH.
The Importance of pH in Different Contexts
The pH of a solution plays a crucial role in numerous applications:
1. Environmental Science:
- Water quality: The pH of water is a key indicator of its quality and health. Significant deviations from neutrality can harm aquatic life and affect ecosystem balance. Acid rain, for example, significantly lowers the pH of water bodies, with devastating consequences for aquatic organisms.
- Soil pH: The pH of soil greatly influences plant growth. Different plants thrive in different pH ranges, and maintaining the optimal pH is essential for healthy plant development.
- Wastewater treatment: pH control is essential in wastewater treatment plants to ensure effective removal of pollutants and to prevent environmental damage.
2. Medicine and Biology:
- Blood pH: Maintaining the pH of blood within a narrow range (around 7.4) is critical for human health. Significant deviations can lead to acidosis or alkalosis, both potentially life-threatening conditions.
- Enzyme activity: Many enzymes function optimally within a specific pH range. Changes in pH can affect their activity and potentially disrupt biological processes.
- Drug delivery: The pH of a drug formulation can affect its absorption, distribution, and efficacy.
3. Industry:
- Food and beverage industry: pH control is essential in food production to maintain food safety, prevent spoilage, and enhance the taste and texture of products.
- Chemical industry: Many industrial processes require precise pH control to optimize reaction rates and product quality.
- Manufacturing: pH control is crucial in various manufacturing processes, including the production of textiles, paper, and pharmaceuticals.
Measuring and Controlling pH
Several methods are used to measure and control pH:
- pH meters: These electronic devices provide a precise measurement of pH by measuring the potential difference between a pH-sensitive electrode and a reference electrode.
- pH indicators: These are substances that change color depending on the pH of the solution. They provide a visual indication of the approximate pH but are less precise than pH meters.
- Titration: This laboratory technique involves adding a solution of known concentration (titrant) to a solution of unknown concentration until the reaction is complete. This allows for precise determination of the pH and concentration of the unknown solution.
- pH control systems: These systems use sensors and actuators to maintain a desired pH level in a process or solution.
Conclusion: The Significance of pH Neutrality and Beyond
The simple question of what constitutes a neutral pH solution – typically 7 at 25°C – opens up a vast landscape of chemical principles and practical applications. Understanding pH is fundamental to various fields, from environmental monitoring and agricultural practices to medical diagnostics and industrial processes. The ability to measure, control, and understand the implications of pH is paramount for ensuring the safety, efficacy, and sustainability of countless processes and systems. Accurate pH measurement and control are crucial for maintaining optimal conditions across diverse applications, highlighting the importance of understanding this fundamental chemical concept. The seemingly simple answer of pH 7 for a neutral solution belies the complex and vital role pH plays in our world.
Latest Posts
Latest Posts
-
What Is The Smallest Unit Of Dna Called
Mar 31, 2025
-
25 Is 4 Of What Number
Mar 31, 2025
-
Is Sand And Water A Solution
Mar 31, 2025
-
Is Hcl A Compound Or Element
Mar 31, 2025
-
All Of The Following Are Characteristics Of Perfect Competition Except
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ph For A Neutral Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
