A Catalyst Lowers The Activation Energy Of A Reaction By
News Leon
Mar 21, 2025 · 6 min read
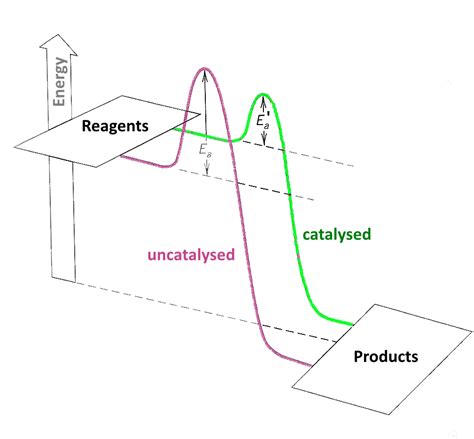
Table of Contents
A Catalyst Lowers the Activation Energy of a Reaction By… Altering the Reaction Pathway
Chemical reactions are the bedrock of the universe, transforming matter from one form to another. However, many reactions, even seemingly simple ones, require a significant input of energy to initiate and proceed. This energy barrier is known as the activation energy (Ea). A catalyst, a remarkable substance, dramatically accelerates the rate of a reaction without being consumed itself, by cleverly lowering this activation energy. But how does a catalyst achieve this feat? This article delves deep into the mechanisms by which catalysts reduce activation energy, exploring various approaches and providing illustrative examples.
Understanding Activation Energy: The Energy Hill
Before diving into the catalyst's role, let's solidify our understanding of activation energy. Imagine a ball perched atop a hill (representing the reactants). To get the ball to roll down the other side (representing the products), you need to give it a push—that push represents the activation energy. The ball won't roll on its own unless it possesses enough energy to overcome the hill's height. Similarly, reactant molecules require sufficient energy to overcome the energy barrier (Ea) and transform into products.
This energy barrier arises because chemical bonds need to be broken in the reactants before new bonds can be formed in the products. Breaking bonds requires energy input. The activation energy is the minimum energy required to reach a transition state, an unstable, high-energy intermediate structure, where bonds are partially broken and partially formed. Once the transition state is reached, the reaction proceeds downhill, releasing energy as the products are formed.
Factors Affecting Activation Energy
Several factors influence the magnitude of activation energy:
- Bond Strength: Stronger bonds require more energy to break, leading to higher activation energy.
- Steric Hindrance: Bulky reactant molecules may hinder the approach of reacting atoms or groups, raising the activation energy.
- Reaction Type: Different reaction types (e.g., SN1, SN2, E1, E2) have inherent differences in their activation energy profiles.
- Solvent Effects: The solvent can stabilize or destabilize reactants and transition states, influencing Ea.
The Catalyst's Role: Providing an Alternate Route
A catalyst doesn't change the overall energy difference between reactants and products (ΔH, the enthalpy change). It doesn't force a reaction that wouldn't otherwise occur thermodynamically. Instead, its magic lies in providing an alternative reaction pathway with a lower activation energy. This alternative route involves the formation of intermediate complexes between the catalyst and the reactants, which then decompose to yield the products and regenerate the catalyst.
How Catalysts Lower Activation Energy: Mechanistic Insights
Catalysts achieve their activation energy reduction through various mechanisms, primarily focusing on:
-
Stabilizing the Transition State: Catalysts interact with the reactants, stabilizing the high-energy transition state. This stabilization lowers the energy of the transition state, effectively reducing the activation energy. Imagine the catalyst as providing scaffolding to support the ball as it climbs the hill, making the climb less steep.
-
Providing an Alternate Reaction Mechanism: A catalyst can facilitate a different reaction mechanism altogether, one with a lower activation energy than the uncatalyzed reaction. This might involve a multi-step process with lower energy barriers for each individual step. The catalyst might, for instance, introduce a new intermediate that reacts more readily with other reactants.
-
Increasing the Frequency of Effective Collisions: In some instances, catalysts enhance the frequency of successful collisions between reactant molecules, which are oriented appropriately for reaction to occur. This doesn't directly reduce Ea, but it increases the reaction rate, achieving a similar outcome.
Examples of Catalytic Action and Activation Energy Reduction
Let's explore a few examples to illustrate these mechanisms:
1. Enzymatic Catalysis: Biological Marvels
Enzymes, biological catalysts, are masters of activation energy reduction. Their intricate three-dimensional structures create a specific binding site (active site) for the substrate (reactant). The active site is precisely engineered to:
- Orient the substrates: Bringing reacting molecules into close proximity and optimal orientation for reaction.
- Strain the substrate bonds: Weakening existing bonds, making them easier to break.
- Stabilize the transition state: Providing specific interactions that lower the transition state energy.
For instance, the enzyme sucrase catalyzes the hydrolysis of sucrose (table sugar) into glucose and fructose. Sucrase binds sucrose in its active site, strategically positioning water molecules to facilitate the bond cleavage, significantly lowering the activation energy compared to the uncatalyzed reaction.
2. Heterogeneous Catalysis: Surface Encounters
Heterogeneous catalysts are solid materials that catalyze reactions involving reactants in different phases (e.g., gas-solid or liquid-solid). The catalytic activity occurs at the catalyst's surface. The mechanism often involves:
- Adsorption: Reactant molecules adsorb (bind) to the catalyst's surface.
- Surface Reactions: Reactants undergo chemical transformations on the surface.
- Desorption: Products desorb from the surface.
A classic example is the Haber-Bosch process for ammonia synthesis (N₂ + 3H₂ → 2NH₃), which uses an iron catalyst. The iron surface adsorbs nitrogen and hydrogen molecules, weakening their triple and single bonds, respectively. This facilitates the formation of N-H bonds, lowering the activation energy for ammonia formation.
3. Homogeneous Catalysis: Solution Partners
Homogeneous catalysts operate in the same phase as the reactants (typically in solution). Their mechanisms often involve the formation of intermediate complexes between the catalyst and the reactants.
For example, many transition metal complexes act as homogeneous catalysts. These complexes can facilitate reactions through:
- Electron Transfer: The catalyst can donate or accept electrons, enabling oxidation or reduction steps with lower activation energies.
- Ligand Coordination: The catalyst can bind to the reactants, changing their electronic structure and promoting bond breaking or formation.
The Importance of Catalyst Specificity and Selectivity
Not all catalysts are created equal. A crucial aspect of catalyst design and selection is specificity and selectivity. A specific catalyst efficiently catalyzes a particular reaction, while a selective catalyst favors the formation of one product over others in a multi-product reaction. This selectivity is often achieved by the catalyst's ability to bind selectively to one reactant or intermediate over others, directing the reaction towards the desired product.
Conclusion: Catalysts – The Unsung Heroes of Chemistry
Catalysts are indispensable in countless chemical processes, from industrial manufacturing to biological systems. Their ability to lower activation energy is pivotal for accelerating reactions and making them economically and practically feasible. Understanding the diverse mechanisms by which catalysts achieve this reduction is crucial for developing new and improved catalytic systems, addressing important challenges in areas such as energy production, environmental remediation, and medicine. The continuing exploration and refinement of catalytic processes promise transformative advances in various fields. Further research is constantly revealing new insights into the intricate details of catalytic action, paving the way for more efficient and sustainable chemical transformations in the future. The subtle dance between catalyst and reactant, a ballet of molecular interactions, remains a captivating subject of study and a key to unlocking many of chemistry's most powerful capabilities.
Latest Posts
Latest Posts
-
Blood Acquires Its Red Color From
Mar 22, 2025
-
The Working People Of France Were Called The
Mar 22, 2025
-
How Do You Balance C2h6 O2 Co2 H2o
Mar 22, 2025
-
Define Heredity Explain The Mechanism Of Hereditary Changes
Mar 22, 2025
-
Number Of Valence Electrons In Copper
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about A Catalyst Lowers The Activation Energy Of A Reaction By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
