1 2 Dibromoethane Condensed Structural Formula
News Leon
Apr 01, 2025 · 5 min read
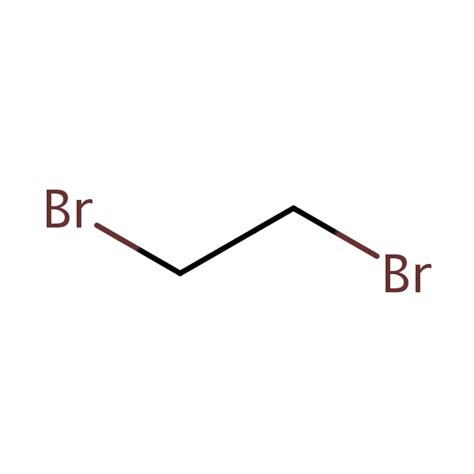
Table of Contents
1,2-Dibromoethane: A Deep Dive into its Condensed Structural Formula, Properties, and Uses
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is a haloalkane with the chemical formula C₂H₄Br₂. Understanding its condensed structural formula is crucial to grasping its properties, applications, and environmental impact. This article provides a comprehensive overview of 1,2-dibromoethane, exploring its structure, characteristics, historical uses, safety concerns, and current relevance.
Understanding the Condensed Structural Formula
The condensed structural formula of 1,2-dibromoethane is BrCH₂CH₂Br. This formula efficiently represents the molecule's connectivity. Let's break it down:
- C₂: Indicates the presence of two carbon atoms.
- H₄: Shows that four hydrogen atoms are bonded to the carbon atoms.
- Br₂: Represents two bromine atoms attached to the carbon atoms.
- CH₂CH₂: This part highlights the arrangement of the carbon atoms, bonded to each other. The subscripts show the number of hydrogen atoms bonded to each carbon.
The full structural formula provides a more visual representation, explicitly showing all bonds:
H H
| |
Br - C - C - Br
| |
H H
Both the condensed and full structural formulas convey the same information: two carbon atoms are linked together, each bonded to two hydrogen atoms and one bromine atom. The bromine atoms are positioned on adjacent carbon atoms, signifying the "1,2" in its name. This specific arrangement of atoms dictates the molecule's properties and behavior.
Key Properties of 1,2-Dibromoethane
The chemical structure of 1,2-dibromoethane directly influences its physical and chemical properties. Key characteristics include:
- Appearance: 1,2-Dibromoethane is a colorless to slightly yellowish liquid at room temperature.
- Odor: It possesses a pungent, sweet odor, which serves as a warning sign of its potential toxicity.
- Density: Denser than water, it has a relatively high density.
- Solubility: It exhibits limited solubility in water, but readily dissolves in many organic solvents.
- Boiling Point: Its boiling point is relatively high due to the presence of polar C-Br bonds and van der Waals forces between molecules.
- Reactivity: The C-Br bonds are relatively weak, making 1,2-dibromoethane susceptible to nucleophilic substitution reactions. This reactivity is critical in some of its past and present applications.
Historical Uses and Applications
Historically, 1,2-dibromoethane had a number of significant applications, most notably as a:
-
Lead Scavenger in Gasoline: Prior to the phasing out of leaded gasoline, EDB was extensively used as a lead scavenger. The lead tetraethyl in leaded gasoline produced lead deposits in engine components. EDB reacted with the lead, forming volatile lead bromide, which was expelled through the exhaust system, preventing lead buildup. This application unfortunately contributed significantly to environmental pollution.
-
Soil Fumigant: EDB's ability to penetrate soil and its toxicity to a broad range of organisms made it a popular soil fumigant in agriculture. It was employed to control various soilborne pests and diseases, boosting crop yields. However, its persistence in soil and groundwater, coupled with its toxicity, led to its eventual ban in many countries.
-
Solvent: Due to its ability to dissolve a variety of organic compounds, 1,2-dibromoethane found limited use as a solvent in certain chemical processes.
Safety Concerns and Environmental Impact
The use of 1,2-dibromoethane is now heavily restricted due to significant safety and environmental concerns. The compound exhibits several harmful effects:
-
Toxicity: EDB is highly toxic, posing risks through inhalation, ingestion, and skin absorption. Exposure can lead to a range of adverse health effects, including central nervous system depression, liver and kidney damage, and potential carcinogenicity.
-
Carcinogenicity: 1,2-Dibromoethane is classified as a human carcinogen by various regulatory agencies. Studies have linked its exposure to an increased risk of several types of cancer.
-
Environmental Persistence: Its persistence in soil and groundwater presents a significant environmental threat. It can contaminate water sources, impacting aquatic life and potentially entering the food chain.
-
Bioaccumulation: The compound can bioaccumulate in organisms, leading to increased concentrations in higher trophic levels.
Current Regulations and Alternatives
Due to its toxicity and environmental persistence, the production and use of 1,2-dibromoethane are now heavily regulated or banned in many countries. Stringent regulations control its handling, storage, and disposal.
Alternatives to EDB have been developed for various applications. These include other soil fumigants, less toxic lead scavengers (although leaded gasoline is largely phased out globally), and alternative solvents depending on the specific application. The search for sustainable and environmentally benign alternatives continues to be an active area of research and development.
Research and Future Directions
While the widespread use of 1,2-dibromoethane is a thing of the past, research continues in related areas:
-
Degradation studies: Scientists are investigating methods for effectively degrading EDB in contaminated soil and water. Bioremediation techniques, using microorganisms to break down the compound, show promise.
-
Development of safer alternatives: The quest for safer and more environmentally friendly alternatives to EDB in various applications is ongoing.
-
Risk assessment and exposure studies: Further research is needed to fully understand the long-term health effects of EDB exposure and to refine risk assessment methodologies.
Conclusion
1,2-Dibromoethane, while possessing useful properties in the past, has been largely replaced due to its significant toxicity and environmental impact. Understanding its condensed structural formula, properties, and history provides crucial insights into its past applications and the ongoing efforts to mitigate its environmental legacy and develop safer alternatives. The stringent regulations surrounding its use underscore the importance of prioritizing environmental protection and human health. The ongoing research in its degradation and the development of safer substitutes showcases a commitment to sustainable practices in chemistry and industry. The story of 1,2-dibromoethane serves as a valuable reminder of the need for careful evaluation of chemical compounds and the responsible development and use of chemicals in various applications. The lessons learned from its past applications should inform future developments in chemical technology, ensuring a safer and more sustainable future.
Latest Posts
Latest Posts
-
Hyposecretion Of The Thyroid Gland In Adulthood
Apr 02, 2025
-
At What Temperature Do The Celsius And Fahrenheit Scales Coincide
Apr 02, 2025
-
2 Is The Only Even Prime Number
Apr 02, 2025
-
Distance Between Earth And Moon In Light Years
Apr 02, 2025
-
Which Of The Following Is Chemically Inert Unreactive
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about 1 2 Dibromoethane Condensed Structural Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
