Write The Iupac Name Of The Compound Shown.
News Leon
Mar 24, 2025 · 6 min read
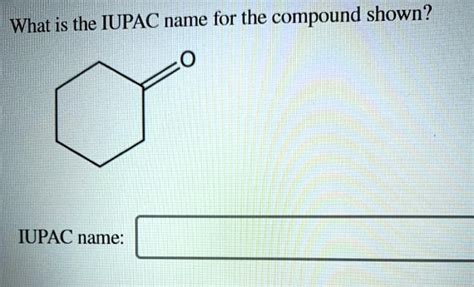
Table of Contents
Decoding Chemical Structures: A Comprehensive Guide to IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) nomenclature is the globally accepted standardized system for naming chemical compounds. This ensures unambiguous communication between chemists worldwide, preventing confusion and misinterpretations that could have serious consequences in various fields, from pharmaceuticals to materials science. Understanding IUPAC nomenclature is crucial for anyone working with chemicals, regardless of their specific area of expertise. This article delves deep into the principles of IUPAC nomenclature, providing a detailed walkthrough of how to name various organic and inorganic compounds, with a particular focus on practical application.
I. Understanding the Fundamentals of IUPAC Nomenclature
Before diving into the complexities of naming specific compounds, it's essential to grasp the core principles underlying the IUPAC system. The system is based on a set of rules and guidelines that allow for the systematic construction of a name from a given chemical structure, and vice versa. Key aspects include:
1. Identifying the Parent Chain or Compound
The foundation of IUPAC naming lies in identifying the parent chain or compound. This is the longest continuous chain of carbon atoms in an organic molecule or the central atom in an inorganic compound. For organic compounds, the parent chain determines the base name (e.g., methane, ethane, propane, etc.). In inorganic compounds, the central atom often dictates the base name (e.g., oxides, chlorides, sulfides, etc.).
2. Identifying Substituents and Functional Groups
Once the parent chain or compound is identified, the next step is to identify any substituents or functional groups attached to it. Substituents are atoms or groups of atoms that replace hydrogen atoms on the parent chain. Functional groups are specific atoms or groups of atoms that determine the chemical properties and reactivity of a molecule. These are crucial for naming and classifying organic compounds. Examples of functional groups include alcohols (-OH), aldehydes (-CHO), ketones (-C=O), carboxylic acids (-COOH), and amines (-NH2).
3. Numbering the Carbon Chain
In organic compounds, the carbon atoms in the parent chain are numbered to indicate the position of substituents or functional groups. Numbering should be done in a way that gives the substituents the lowest possible numbers. If there are multiple substituents, alphabetical order is typically used to resolve ambiguities.
4. Applying IUPAC Rules for Prefix, Infix, and Suffix
The IUPAC name is constructed using prefixes, infixes, and suffixes, each representing specific structural features.
- Prefixes: Indicate the presence and position of substituents (e.g., methyl, ethyl, chloro, bromo). Prefixes are placed before the base name.
- Infixes: Indicate the presence of double or triple bonds (e.g., -en- for double bonds, -yn- for triple bonds). These are inserted within the base name.
- Suffixes: Indicate the presence of functional groups (e.g., -ol for alcohols, -al for aldehydes, -one for ketones, -oic acid for carboxylic acids). Suffixes are placed at the end of the base name.
5. Using Alphabetical Order and Multipliers
When multiple substituents are present, they are listed alphabetically, ignoring prefixes like di-, tri-, tetra-, etc., unless they are part of a complex substituent name. Prefixes like di-, tri-, tetra- are used to indicate the number of times a particular substituent appears.
II. Naming Alkanes, Alkenes, and Alkynes
Alkanes, alkenes, and alkynes are hydrocarbons—organic compounds containing only carbon and hydrogen atoms. Their IUPAC names are constructed based on the number of carbon atoms and the presence of single, double, or triple bonds.
- Alkanes: These contain only single bonds. The base names are methane (1 carbon), ethane (2 carbons), propane (3 carbons), butane (4 carbons), and so on.
- Alkenes: These contain at least one carbon-carbon double bond. The suffix "-ene" is used, and the position of the double bond is indicated by a number.
- Alkynes: These contain at least one carbon-carbon triple bond. The suffix "-yne" is used, and the position of the triple bond is indicated by a number.
III. Naming Branched-Chain Alkanes
When dealing with branched-chain alkanes, the longest continuous carbon chain is identified as the parent chain. Branches are treated as substituents, named using the alkyl group prefixes (e.g., methyl, ethyl, propyl), and their positions are indicated by numbers.
IV. Naming Compounds with Functional Groups
Compounds containing functional groups are named by identifying the parent chain, locating the functional group, and using the appropriate suffix. The position of the functional group is indicated by a number. For example:
- Alcohols (-OH): The suffix "-ol" is used.
- Aldehydes (-CHO): The suffix "-al" is used.
- Ketones (-C=O): The suffix "-one" is used.
- Carboxylic acids (-COOH): The suffix "-oic acid" is used.
- Amines (-NH2): The suffix "-amine" is used.
V. Naming Cyclic Compounds
Cyclic compounds (compounds with rings of atoms) have specific IUPAC naming rules. The prefix "cyclo-" is added to the alkane name corresponding to the number of carbon atoms in the ring. For example, cyclopropane (3-carbon ring), cyclobutane (4-carbon ring), cyclopentane (5-carbon ring), etc.
VI. Naming Aromatic Compounds
Aromatic compounds contain a benzene ring (a six-membered ring with alternating single and double bonds). Substituted benzene derivatives are named using the prefixes ortho- (1,2-), meta- (1,3-), and para- (1,4-) to indicate the relative positions of the substituents on the ring. More complex aromatic compounds may require more elaborate naming conventions.
VII. Naming Inorganic Compounds
Inorganic compounds are named using different conventions compared to organic compounds. The name generally reflects the elements present and their oxidation states. For simple binary compounds (compounds composed of two elements), the more electropositive element is named first, followed by the more electronegative element with the suffix "-ide."
VIII. Advanced IUPAC Nomenclature: Stereochemistry and Isomerism
The IUPAC nomenclature system also incorporates rules for describing the stereochemistry (three-dimensional arrangement of atoms) of molecules. This includes specifying the configuration of chiral centers (using R/S notation), the geometry of double bonds (using cis/trans or E/Z notation), and other aspects of molecular structure. Understanding these aspects is essential for accurate and complete chemical communication, especially in fields like pharmaceuticals where subtle differences in stereochemistry can significantly impact biological activity.
IX. Practical Application and Example
Let's consider a specific example to illustrate the application of IUPAC nomenclature. Suppose we have a compound with the following structure:
[Insert a chemical structure image here. For example, a simple branched alkane with methyl and ethyl substituents].
To name this compound:
- Identify the longest carbon chain: This forms the parent chain.
- Number the carbon atoms: Start from the end closest to the substituents to give them the lowest numbers.
- Identify the substituents: Determine the type and position of each substituent (e.g., methyl, ethyl).
- Construct the name: Use the prefixes for the substituents, the base name for the parent chain (based on its number of carbons), and the correct numbering to indicate the substituent positions. For example, if the structure shows a 5-carbon chain with a methyl group on carbon 2 and an ethyl group on carbon 3, the IUPAC name would be 3-ethyl-2-methylpentane.
X. Conclusion
Mastering IUPAC nomenclature is a crucial skill for anyone involved in chemistry. It's a systematic and logical approach to naming chemical compounds, ensuring clear and unambiguous communication in all areas of chemistry. While the rules may seem complex at first, consistent practice and a methodical approach can lead to a strong understanding of this essential aspect of the chemical sciences. The ability to accurately name and interpret chemical structures using IUPAC nomenclature is fundamental to success in numerous scientific disciplines and industries. Remember to practice regularly, referring to IUPAC guidelines and examples to solidify your understanding and build proficiency in this vital field.
Latest Posts
Latest Posts
-
Draw A Bond Line Structure For The Following Compound
Mar 27, 2025
-
How Many Moles Of Water In 1 Liter
Mar 27, 2025
-
A Plastic Rod Has Been Bent
Mar 27, 2025
-
2000 Mg Is How Many G
Mar 27, 2025
-
What Is 4 To The 5th Power
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Write The Iupac Name Of The Compound Shown. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
