Write The Complete Ground-state Electron Configuration Of Aluminum
News Leon
Mar 22, 2025 · 6 min read
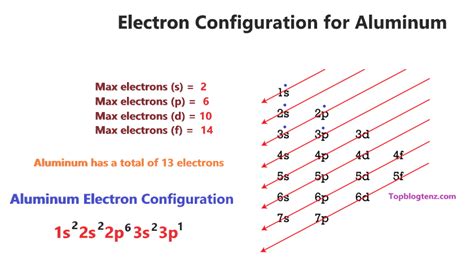
Table of Contents
Unveiling the Secrets of Aluminum: A Deep Dive into its Ground-State Electron Configuration
Aluminum, a ubiquitous metal found in everything from soda cans to aircraft parts, holds a fascinating place in the periodic table. Understanding its atomic structure, particularly its ground-state electron configuration, is key to appreciating its unique properties and reactivity. This comprehensive guide will delve into the intricacies of aluminum's electron configuration, explaining the underlying principles and showcasing its relevance in various scientific fields.
Understanding Electron Configuration
Before we dive into the specifics of aluminum, let's establish a foundational understanding of electron configuration. An atom's electron configuration describes how electrons are arranged within its electron shells and subshells. This arrangement is governed by several fundamental principles:
-
The Aufbau Principle: Electrons fill the lowest energy levels first. Think of it as electrons seeking the most stable and energetically favorable positions within the atom.
-
The Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins. This ensures that no two electrons within an atom share the same set of quantum numbers.
-
Hund's Rule: Electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration.
These principles are crucial for predicting the electron configuration of any element, including aluminum.
Delving into Aluminum's Atomic Structure
Aluminum (Al) boasts an atomic number of 13, meaning it has 13 protons in its nucleus and, in its neutral state, 13 electrons surrounding the nucleus. To determine its ground-state electron configuration, we follow the Aufbau principle and fill the electron shells and subshells according to their increasing energy levels.
The Shell Model and Subshells
The electrons are arranged in shells (principal energy levels), denoted by the principal quantum number (n). Each shell can accommodate a specific maximum number of electrons:
- Shell 1 (n=1): Holds a maximum of 2 electrons.
- Shell 2 (n=2): Holds a maximum of 8 electrons.
- Shell 3 (n=3): Holds a maximum of 18 electrons. And so on...
Within each shell, there are subshells (s, p, d, f), which are characterized by their angular momentum quantum number (l). Each subshell can hold a certain number of electrons:
- s subshell (l=0): Holds a maximum of 2 electrons.
- p subshell (l=1): Holds a maximum of 6 electrons.
- d subshell (l=2): Holds a maximum of 10 electrons.
- f subshell (l=3): Holds a maximum of 14 electrons.
Building Aluminum's Electron Configuration
Now, let's build aluminum's electron configuration step-by-step:
-
Shell 1 (n=1): The lowest energy level is the 1s subshell, which can hold 2 electrons. So, we fill it completely: 1s²
-
Shell 2 (n=2): Next, we move to shell 2. We first fill the 2s subshell with 2 electrons: 2s². Then we fill the 2p subshell, which can hold up to 6 electrons. However, aluminum only has 3 more electrons to place, so we fill three of the six available orbitals in the 2p subshell: 2p⁶.
-
Shell 3 (n=3): After filling shell 2, we have 3 electrons remaining. These will fill the lowest energy subshell in shell 3, which is the 3s subshell: 3s¹
Therefore, the complete ground-state electron configuration of aluminum is: 1s²2s²2p⁶3s¹.
Orbital Diagrams and Electron Spin
A more detailed representation of the electron configuration can be achieved using orbital diagrams. These diagrams illustrate the arrangement of electrons within individual orbitals, showing electron spin using arrows (↑ and ↓). For Aluminum:
- 1s: ↑↓
- 2s: ↑↓
- 2p: ↑↓ ↑↓ ↑↓
- 3s: ↑
This diagram visually confirms that each orbital is filled according to Hund's rule and the Pauli exclusion principle.
Significance of Aluminum's Electron Configuration
Understanding aluminum's electron configuration is critical for understanding its chemical behavior and properties:
-
Valence Electrons: The outermost electrons, those in the 3s orbital, are the valence electrons. These electrons are involved in chemical bonding and determine aluminum's reactivity. With only one valence electron, aluminum readily loses this electron to form a stable +3 ion (Al³⁺). This tendency explains its metallic character and its ability to form ionic compounds.
-
Metallic Bonding: The ease with which aluminum loses its valence electron contributes to its strong metallic bonding. The valence electrons become delocalized, forming a "sea" of electrons that holds the positively charged aluminum ions together. This explains aluminum's excellent electrical and thermal conductivity.
-
Reactivity and Chemical Properties: The single valence electron makes aluminum relatively reactive, particularly with oxidizing agents. However, a thin layer of aluminum oxide (Al₂O₃) forms on its surface, protecting the underlying metal from further oxidation, hence its relatively high corrosion resistance.
-
Applications: Aluminum's unique properties, directly linked to its electron configuration, account for its widespread use in various applications. Its lightweight nature, strength, corrosion resistance, and conductivity make it ideal for aircraft manufacturing, packaging, electrical wiring, and numerous other industries.
Aluminum and the Periodic Table
Aluminum's position in the periodic table, specifically in Group 13 (or IIIA), reflects its electron configuration. Group 13 elements are characterized by having three valence electrons in their outermost shell. This shared characteristic explains the similarities in their chemical behavior. Aluminum shares similarities with other group 13 elements like boron (B), gallium (Ga), indium (In), and thallium (Tl), although differences exist due to variations in their inner electron configurations and atomic sizes.
Advanced Concepts and Further Exploration
The discussion above provides a foundational understanding of aluminum's ground-state electron configuration. However, a deeper exploration of quantum mechanics, including quantum numbers and orbital shapes, can provide a more comprehensive appreciation of the underlying physics governing electron behavior within the atom.
Further investigations could include:
-
Excited States: While the ground-state configuration represents the lowest energy state, aluminum can exist in excited states where electrons occupy higher energy levels. Understanding these excited states is crucial for explaining aluminum's spectral lines.
-
Ionization Energies: The energy required to remove electrons from aluminum, sequentially, are linked directly to the electron configuration. The first ionization energy (removing the 3s electron) is relatively low, while subsequent ionization energies progressively increase as electrons are removed from more tightly bound inner shells.
-
Electron Affinity: Although aluminum doesn't readily accept electrons, its electron affinity (the energy change associated with adding an electron) can be calculated and provides further insights into its chemical reactivity.
Conclusion
Aluminum's ground-state electron configuration (1s²2s²2p⁶3s¹) is the cornerstone for understanding its properties and behavior. From its metallic bonding to its reactivity and widespread applications, the arrangement of its 13 electrons dictates its significant role in various scientific and technological fields. This comprehensive exploration provides a solid foundation for anyone seeking a deeper understanding of this fundamental aspect of aluminum's atomic structure and its implications in the wider world. The knowledge gained here underscores the critical relationship between an element's electron configuration and its macroscopic properties, illustrating the power of atomic-level understanding in comprehending the material world around us.
Latest Posts
Latest Posts
-
How To Write Letter To The Bank
Mar 23, 2025
-
How Long Does A Cow Sleep
Mar 23, 2025
-
A Skier Is Pulled By A Tow Rope
Mar 23, 2025
-
Is A Candle Burning A Physical Or Chemical Change
Mar 23, 2025
-
Who Is The Maker Of A Promissory Note
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Write The Complete Ground-state Electron Configuration Of Aluminum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
