Why Water Is Called A Universal Solvent
News Leon
Mar 17, 2025 · 6 min read
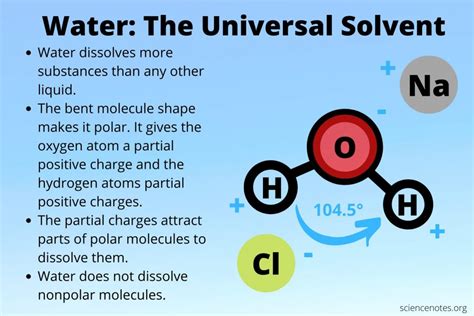
Table of Contents
Why Water Is Called the Universal Solvent: A Deep Dive
Water. It's the lifeblood of our planet, essential for all known forms of life. But beyond its biological importance, water possesses a unique characteristic that makes it incredibly significant in chemistry and many other fields: it's often called the universal solvent. This isn't to say water dissolves everything – far from it – but its ability to dissolve a remarkably wide range of substances far surpasses that of most other solvents. Understanding why water earns this title requires exploring its molecular structure and the powerful forces that govern its interactions with other molecules.
The Polar Nature of Water: The Key to its Solvency
At the heart of water's solvent power lies its polarity. A water molecule (H₂O) consists of two hydrogen atoms covalently bonded to a single oxygen atom. Oxygen is significantly more electronegative than hydrogen, meaning it attracts the shared electrons in the covalent bonds more strongly. This creates an uneven distribution of charge within the molecule. The oxygen atom carries a partial negative charge (δ-), while each hydrogen atom carries a partial positive charge (δ+). This uneven distribution makes water a polar molecule, possessing a positive and a negative end, much like a tiny magnet.
How Polarity Drives Dissolution
This polarity is the driving force behind water's ability to dissolve many substances. Polar molecules, or ions (charged particles), readily interact with water molecules through a process called solvation or hydration. The positive ends of water molecules are attracted to the negative ions or the negatively charged parts of polar molecules, while the negative ends of water molecules are attracted to the positive ions or the positively charged parts of polar molecules. This attractive force surrounds the ions or molecules, effectively pulling them apart from each other and into the water.
Types of Substances Water Dissolves Well:
Water excels at dissolving a variety of substances, including:
1. Ionic Compounds:
Ionic compounds, like table salt (NaCl), are composed of positively charged ions (cations) and negatively charged ions (anions) held together by strong electrostatic forces. When salt is added to water, the polar water molecules surround the sodium (Na+) and chloride (Cl-) ions. The partial negative charges on the oxygen atoms of water molecules attract the positively charged sodium ions, while the partial positive charges on the hydrogen atoms attract the negatively charged chloride ions. This interaction weakens the ionic bonds in the salt crystal, causing the ions to separate and become surrounded by water molecules, a process called dissociation. The resulting solution is a homogeneous mixture of freely moving sodium and chloride ions dissolved in water.
2. Polar Molecules:
Polar molecules, like sugar (sucrose), contain polar bonds with an uneven distribution of charge. Similar to ionic compounds, the positive and negative ends of water molecules interact with the oppositely charged regions of the sugar molecules. These interactions weaken the intermolecular forces holding the sugar molecules together, causing them to dissolve and disperse throughout the water. This process is often described as like dissolves like, emphasizing the tendency of polar solvents to dissolve polar solutes.
3. Some Gases:
Even some gases, like oxygen (O₂) and carbon dioxide (CO₂), dissolve in water to some extent, although their solubility is generally lower than that of ionic or polar compounds. While these gases are nonpolar, their interaction with water is mediated by weak intermolecular forces such as van der Waals forces. This explains why aquatic life can breathe oxygen dissolved in water.
Why Water Isn't a Truly Universal Solvent:
Despite its impressive ability, it’s crucial to understand that water isn't a truly universal solvent. Many substances are largely insoluble in water, including:
1. Nonpolar Compounds:
Nonpolar compounds, like oils and fats, lack a significant charge separation. Since water molecules are strongly attracted to each other through hydrogen bonding (a special type of dipole-dipole interaction between a hydrogen atom and an electronegative atom like oxygen), they have little incentive to interact with nonpolar molecules. The strong hydrogen bonds within water prevent it from effectively disrupting the weak intermolecular forces holding nonpolar molecules together, resulting in limited solubility.
2. Some Ionic Compounds:
While many ionic compounds are highly soluble in water, some are sparingly soluble or insoluble. This depends on several factors, including the strength of the ionic bonds within the compound and the relative strengths of the interactions between the ions and water molecules. The solubility of ionic compounds can also be affected by the presence of other ions in the solution, a concept explored in solubility product equilibria.
3. Macromolecules:
Large molecules such as proteins and DNA exhibit complex structures and various types of interactions within their structures. While some parts of these molecules may be polar and thus interact with water, other parts may be nonpolar, leading to limited solubility or a requirement for specialized conditions to ensure dissolution.
Factors Affecting Water's Solvent Power:
Several factors influence the extent to which water can dissolve different substances:
-
Temperature: Higher temperatures generally increase the solubility of most solids and gases in water. Increased kinetic energy allows water molecules to more effectively overcome the attractive forces holding the solute particles together.
-
Pressure: Pressure primarily affects the solubility of gases in water. Higher pressures increase the solubility of gases, as demonstrated by Henry's Law.
-
pH: The acidity or alkalinity of the solution (pH) can significantly influence the solubility of certain substances. Changes in pH can alter the charge on molecules, affecting their interactions with water.
The Importance of Water as a Solvent in Biological Systems:
Water's solvent properties are absolutely crucial for life. Many biological processes depend on water's ability to dissolve and transport nutrients, hormones, and other essential molecules. Blood plasma, for example, is primarily water, acting as a solvent for carrying oxygen, glucose, and other vital substances throughout the body. Similarly, intracellular fluid within cells dissolves numerous biomolecules, allowing for essential metabolic reactions to occur.
Water's Role in Various Industries:
Beyond its biological role, water’s solvent power is exploited in numerous industries:
-
Cleaning: Water is a crucial component of many cleaning solutions, effectively dissolving dirt, grease, and other contaminants.
-
Pharmaceuticals: Water is used extensively in the production and formulation of drugs, as a solvent for active ingredients and as a component of various pharmaceutical preparations.
-
Food Processing: Water is essential for dissolving ingredients, creating mixtures, and cleaning equipment in the food processing industry.
-
Manufacturing: Many industrial processes rely on water as a solvent for cleaning, mixing, and other applications.
Conclusion:
Water's reputation as the "universal solvent" is well-deserved, given its exceptional ability to dissolve a wide range of substances. However, it's crucial to remember that this ability is not absolute. Understanding the interplay between water's polar nature, the properties of the solute, and environmental factors provides a more nuanced understanding of its solvent properties and its profound impact on biological and industrial systems. The ongoing research into water's unique characteristics continues to reveal new insights into its critical role in shaping our world. The seemingly simple water molecule, with its remarkable ability to dissolve so much, remains a subject of fascinating scientific inquiry, constantly highlighting the intricate beauty and functionality of the natural world.
Latest Posts
Latest Posts
-
Dover Beach By Matthew Arnold Explanation
Mar 18, 2025
-
How Many Oxygen Molecules Can One Hemoglobin Carry
Mar 18, 2025
-
Which Of The Following Is Not A Form Of Precipitation
Mar 18, 2025
-
Which Statement About Natural Selection Is True
Mar 18, 2025
-
Which Chamber Of Heart Has Thickest Wall
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Why Water Is Called A Universal Solvent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
