Why Sigma Bond Is Stronger Than Pi Bond
News Leon
Mar 22, 2025 · 6 min read
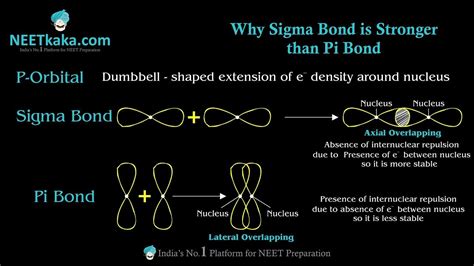
Table of Contents
Why Sigma (σ) Bonds Are Stronger Than Pi (π) Bonds: A Deep Dive into Chemical Bonding
The world of chemistry is built upon the fundamental concept of bonding, and within that, the distinction between sigma (σ) and pi (π) bonds is crucial. While both contribute to the overall strength of a covalent bond, sigma bonds consistently exhibit greater strength. Understanding why this is true requires a delve into the intricacies of atomic orbital overlap and electron density distribution. This article will explore the fundamental differences between sigma and pi bonds, explaining the reasons behind sigma's superior strength and exploring the implications of this difference in various chemical contexts.
Understanding Sigma (σ) and Pi (π) Bonds
Before delving into the strength comparison, let's establish a clear understanding of what defines each bond type. Both sigma and pi bonds result from the overlap of atomic orbitals, enabling atoms to share electrons and achieve a more stable electron configuration. However, the manner of this overlap is key to their differences:
Sigma (σ) Bonds: Head-on Overlap
A sigma bond is formed by the direct, head-on overlap of atomic orbitals. This means the electron density is concentrated along the internuclear axis – the imaginary line connecting the centers of the two bonded atoms. This type of overlap leads to a cylindrically symmetrical electron cloud, maximizing electron density between the nuclei. This strong, direct interaction significantly strengthens the bond. Sigma bonds are the strongest type of covalent bond and are always present in a single bond. They are formed from the overlap of s orbitals with s orbitals, s orbitals with p orbitals, or even hybridized orbitals.
Pi (π) Bonds: Sideways Overlap
Pi bonds are formed by the sideways overlap of atomic orbitals. Unlike sigma bonds, the electron density is concentrated above and below the internuclear axis, rather than directly between the nuclei. This sideways overlap results in a region of electron density that is shaped like two parallel lobes, one above and one below the sigma bond. Pi bonds are weaker than sigma bonds because the sideways overlap is less effective at concentrating electron density between the nuclei, compared to the direct head-on overlap of sigma bonds. Pi bonds are always formed in addition to a sigma bond, forming double or triple bonds.
Why Sigma Bonds Are Stronger: A Comparative Analysis
The superior strength of sigma bonds stems from several factors:
1. Greater Overlap and Electron Density:
The head-on overlap in sigma bonds leads to a significantly larger region of overlap between the atomic orbitals compared to the sideways overlap in pi bonds. This results in a much higher electron density concentrated between the nuclei. This higher electron density strengthens the electrostatic attraction between the positively charged nuclei and the negatively charged electrons, leading to a stronger bond.
2. Stronger Electrostatic Attraction:
The concentrated electron density in the sigma bond creates a stronger electrostatic attraction between the bonded atoms. This attraction directly contributes to the bond's strength, pulling the nuclei closer together. This effect is less pronounced in pi bonds because the electron density is distributed over a larger region, reducing the overall electrostatic attraction.
3. Reduced Electron Repulsion:
The cylindrical symmetry of the sigma bond minimizes electron-electron repulsion. The electrons are distributed more evenly, minimizing their mutual repulsion. In contrast, the electron density in pi bonds is concentrated in two separate lobes, potentially leading to increased electron-electron repulsion and thus a slightly weaker bond.
4. Bond Length and Strength Correlation:
Generally, shorter bond lengths correlate with stronger bonds. Sigma bonds, because of their greater overlap, exhibit shorter bond lengths compared to pi bonds in a multiple bond. This shorter distance contributes to the stronger electrostatic attraction between the nuclei, further solidifying the sigma bond's strength advantage.
Implications of Sigma and Pi Bond Strength Differences
The difference in strength between sigma and pi bonds has significant implications in various aspects of chemistry:
1. Bond Rotation:
Sigma bonds allow for free rotation around the internuclear axis. This is because the electron density is symmetric around the axis, and rotation doesn't significantly disrupt the overlap. In contrast, rotation around a pi bond requires breaking the pi bond, as rotating the atoms would disrupt the sideways overlap. This restricted rotation influences the molecule's shape and reactivity.
2. Reactivity:
The relative strength of sigma and pi bonds significantly impacts a molecule's reactivity. Pi bonds, being weaker, are more susceptible to breaking during chemical reactions. This is why molecules with multiple bonds often undergo addition reactions, where the pi bond breaks and new atoms are added. Sigma bonds, being stronger, are typically more resistant to breaking, often requiring more energy to disrupt.
3. Bond Energy:
The bond energy is the energy required to break a chemical bond. Sigma bonds have higher bond energies than pi bonds. This reflects the strength of the bond, where higher energy means a stronger, more stable bond. This difference in bond energy plays a crucial role in determining the thermodynamic stability of molecules.
4. Spectroscopic Analysis:
Differences in bond strength and electron distribution between sigma and pi bonds are reflected in the molecule's spectroscopic properties. Techniques like infrared (IR) and Raman spectroscopy can distinguish between the vibrational frequencies associated with sigma and pi bonds, providing insights into the structure and composition of molecules.
5. Organic Chemistry and Reactivity:
In organic chemistry, understanding the relative strengths of sigma and pi bonds is crucial for predicting and explaining the reactivity of molecules. The presence of pi bonds often dictates the reaction mechanisms and product formations. The ease of pi bond breakage influences the susceptibility of a molecule to various reactions like electrophilic addition, nucleophilic addition, and free-radical reactions.
Advanced Considerations: Hybridization and Bond Strength
The concept of hybridization adds another layer of complexity. Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals that participate in bonding. These hybrid orbitals, like sp, sp², and sp³, often participate in sigma bonding, further influencing the overall bond strength. For example, sp hybridized orbitals, which have higher s-character, form stronger sigma bonds than sp³ hybridized orbitals. This is because s orbitals are inherently closer to the nucleus, leading to stronger interactions with other orbitals.
Conclusion: The Predominant Strength of Sigma Bonds
In conclusion, the superior strength of sigma bonds compared to pi bonds stems from the fundamental differences in their orbital overlap and electron density distribution. The head-on overlap in sigma bonds leads to stronger electrostatic attraction, higher electron density between the nuclei, and reduced electron-electron repulsion, all contributing to a stronger and more stable bond. Understanding this difference is crucial for comprehending various aspects of chemical bonding, reactivity, and molecular properties, impacting fields ranging from organic chemistry and materials science to biochemistry and drug discovery. The strength of sigma bonds is a cornerstone principle upon which much of our understanding of the molecular world is built.
Latest Posts
Latest Posts
-
Complete The Sentence With The Correct Form Of The Word
Mar 22, 2025
-
What Causes Movement Along The Demand Curve
Mar 22, 2025
-
A Cat Dozes On A Stationary Merry Go Round
Mar 22, 2025
-
What Is The Name For N2o5
Mar 22, 2025
-
An Isolated Conductor Has A Net Charge Of
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Why Sigma Bond Is Stronger Than Pi Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
