Why Are Metals Good Conductors Of Heat
News Leon
Mar 18, 2025 · 6 min read
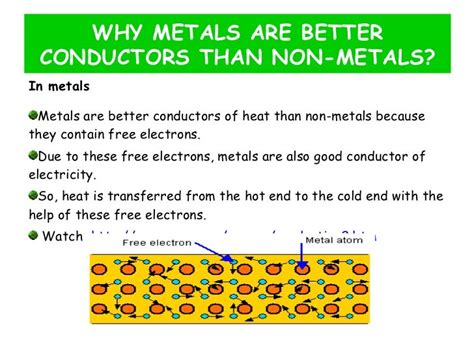
Table of Contents
Why Are Metals Good Conductors of Heat? Unveiling the Secrets of Thermal Conductivity
Metals are renowned for their excellent ability to conduct heat, a property crucial in numerous applications, from cooking utensils to heat sinks in electronics. But why exactly are metals such efficient heat conductors? This article delves deep into the microscopic world to unravel the secrets behind this fundamental property of metals, exploring the role of free electrons, lattice vibrations, and other contributing factors. We will also touch upon the variations in thermal conductivity across different metals and the implications of this property in various technologies.
The Role of Free Electrons: The Key Players in Thermal Conduction
The most significant factor contributing to the high thermal conductivity of metals is the presence of free electrons. Unlike in insulators where electrons are tightly bound to their respective atoms, metals possess a "sea" of delocalized electrons that are not associated with any particular atom. These free electrons are crucial for both electrical and thermal conductivity.
The Drude Model: A Simple Explanation
A simple yet insightful model for understanding this phenomenon is the Drude model. This classical model imagines the free electrons as a gas moving randomly within the metal lattice. When a temperature difference is applied across the metal, the electrons in the hotter region possess higher kinetic energy. These high-energy electrons collide with and transfer their energy to the lower-energy electrons in the colder region, effectively transferring heat throughout the material. This process is incredibly efficient due to the high mobility and density of free electrons in metals.
Beyond the Drude Model: Quantum Mechanics Offers a More Complete Picture
While the Drude model provides a basic understanding, a more accurate description requires quantum mechanics. The quantum mechanical picture considers the electrons occupying energy levels within the metal's electronic band structure. The application of heat leads to electrons jumping to higher energy levels, and these excited electrons contribute to the heat transfer process. This process is more complex than the classical Drude model, but it emphasizes the pivotal role of electron behavior in thermal conductivity.
Lattice Vibrations: A Secondary Contributor to Heat Transfer
While free electrons are the primary contributors, lattice vibrations, also known as phonons, also play a role in heat conduction in metals. These vibrations represent the collective motion of atoms in the metal lattice. When one part of the metal is heated, the atoms in that region vibrate more vigorously, transmitting their kinetic energy to neighboring atoms through collisions. This process contributes to heat transfer, albeit less significantly than electron conduction.
The Interplay Between Electrons and Phonons
The interaction between free electrons and phonons is complex. While both contribute to heat conduction, they can also scatter each other, hindering the overall efficiency of heat transfer. This scattering effect becomes more pronounced at higher temperatures where both electrons and phonons are more energetic and experience more frequent collisions.
Factors Affecting Thermal Conductivity in Metals
The thermal conductivity of a metal is not a fixed value but rather depends on several factors:
1. Temperature: The Temperature Dependence of Thermal Conductivity
The thermal conductivity of most metals generally decreases with increasing temperature. This is because the increased thermal energy leads to more frequent and stronger scattering of both electrons and phonons, thus reducing their ability to effectively transfer heat. However, there are exceptions to this general trend, especially at very low temperatures where other effects become dominant.
2. Purity: Impurities Reduce Thermal Conductivity
The presence of impurities within the metal lattice significantly reduces thermal conductivity. Impurities act as scattering centers for both electrons and phonons, disrupting the efficient flow of heat. Therefore, higher purity metals generally exhibit better thermal conductivity.
3. Crystal Structure: The Influence of Atomic Arrangement
The crystal structure of a metal also influences its thermal conductivity. Metals with highly ordered crystal structures typically have higher thermal conductivity than those with disordered structures. This is because a well-ordered structure facilitates a more efficient pathway for heat transfer.
4. Alloying: The Complex Effects of Alloy Composition
Alloying, the process of mixing different metals, can have a complex effect on thermal conductivity. The addition of alloying elements can either increase or decrease thermal conductivity depending on the specific elements and their concentrations. Some alloying elements can enhance scattering, reducing conductivity, while others may have minimal effect or even improve it in certain cases.
Comparison of Thermal Conductivity Across Different Metals
Different metals exhibit varying levels of thermal conductivity due to differences in their electronic structure, crystal structure, and other factors. Some notable examples include:
-
Silver (Ag): Renowned for its exceptionally high thermal conductivity, making it ideal for applications requiring efficient heat dissipation.
-
Copper (Cu): Another excellent conductor, widely used in electrical wiring and heat exchangers due to its high conductivity and relatively low cost.
-
Aluminum (Al): Possesses good thermal conductivity and a low density, making it suitable for applications where lightweight yet efficient heat transfer is needed.
-
Iron (Fe): Exhibits moderate thermal conductivity, widely used in various structural and engineering applications.
-
Lead (Pb): Shows relatively low thermal conductivity compared to other metals.
These variations highlight the importance of selecting the appropriate metal based on the specific needs of the application.
Applications Leveraging the High Thermal Conductivity of Metals
The excellent heat conductivity of metals finds wide-ranging applications across various industries:
1. Cooking Utensils: Efficient Heat Transfer for Even Cooking
The high thermal conductivity of metals like aluminum and copper is crucial for the design of cooking utensils. These metals efficiently transfer heat from the stovetop to the food, ensuring even cooking and preventing hot spots.
2. Heat Exchangers: Efficient Heat Transfer in Industrial Processes
Heat exchangers rely on the high thermal conductivity of metals to transfer heat between two fluids. These devices are vital in various industrial processes, such as power generation, refrigeration, and chemical processing.
3. Electronics Cooling: Preventing Overheating in Electronic Devices
The miniaturization of electronic devices necessitates efficient heat dissipation to prevent overheating. Metals like aluminum and copper are used in heat sinks to effectively conduct heat away from electronic components, ensuring their proper functioning.
4. Building Materials: Thermal Management in Construction
Metals, especially those with high thermal conductivity, can also be incorporated into building materials to aid in thermal management. However, their high conductivity can also be a disadvantage in some applications, requiring consideration of insulation properties.
Conclusion: Understanding and Harnessing the Power of Thermal Conductivity in Metals
The exceptional thermal conductivity of metals stems primarily from the presence of free electrons that efficiently transfer energy throughout the material. While lattice vibrations also play a role, the dominant contribution of free electrons explains the superior heat transfer capabilities of metals compared to other material classes. The thermal conductivity of a metal is not solely determined by its elemental composition but also depends on temperature, purity, crystal structure, and alloying. Understanding these factors is vital for material selection in various engineering applications. The high thermal conductivity of metals remains a crucial property exploited extensively in numerous technologies, from everyday cooking utensils to sophisticated industrial processes and electronic devices. Continuous research into new alloys and metal processing techniques aims to further refine and enhance the already impressive thermal conductivity properties of metals, paving the way for even more innovative applications in the future.
Latest Posts
Latest Posts
-
How Many Chromosomes Are Present During Prophase
Mar 19, 2025
-
How Many Yards Are In A 1 4 Mile
Mar 19, 2025
-
Label The Structures Of A Nephron In The Figure
Mar 19, 2025
-
What Type Of Joint Is Between The Sternum And Rib
Mar 19, 2025
-
Receptacle Is Part Of The Four Whorls
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Why Are Metals Good Conductors Of Heat . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
