Which Of The Following Statements Is Not True About Enzymes
News Leon
Mar 18, 2025 · 5 min read
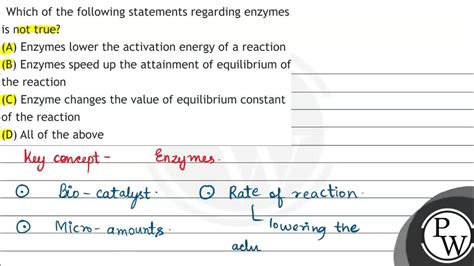
Table of Contents
Which of the Following Statements is NOT True About Enzymes?
Enzymes are biological catalysts that accelerate chemical reactions within living organisms. Understanding their properties and limitations is crucial in various fields, from medicine to industrial biotechnology. This comprehensive article will explore common misconceptions about enzymes and definitively answer the question: which statement regarding enzymes is not true? We’ll delve into the intricacies of enzyme function, exploring their specificity, regulation, and susceptibility to environmental factors.
Understanding the Fundamental Nature of Enzymes
Before we can identify false statements about enzymes, we need a solid understanding of what they are and how they function. At their core, enzymes are primarily proteins (although some RNA molecules also exhibit catalytic activity, known as ribozymes). Their three-dimensional structure is vital to their function. This intricate structure contains a specific region called the active site, where the substrate (the molecule the enzyme acts upon) binds.
Key Characteristics of Enzymes:
-
Specificity: Enzymes exhibit a high degree of specificity, meaning they only catalyze specific reactions with specific substrates. This specificity is due to the precise shape and chemical properties of the active site. The "lock and key" model and the more refined "induced fit" model illustrate this principle.
-
Catalytic Efficiency: Enzymes significantly increase the rate of reactions, often by several orders of magnitude, without being consumed in the process. They achieve this by lowering the activation energy—the energy barrier that must be overcome for a reaction to proceed.
-
Regulation: Enzyme activity is tightly regulated within cells to ensure that metabolic pathways function efficiently and are responsive to changing cellular conditions. This regulation can involve various mechanisms, including allosteric regulation, covalent modification, and feedback inhibition.
-
Sensitivity to Environmental Factors: Enzyme activity is sensitive to various environmental factors, including temperature, pH, and the presence of inhibitors or activators. Optimal conditions vary depending on the specific enzyme. Extreme conditions can denature the enzyme, destroying its catalytic activity.
Common Misconceptions about Enzymes:
Now that we have established the foundational properties of enzymes, let's examine some commonly held beliefs that are not entirely accurate:
1. "Enzymes are consumed during the reaction they catalyze." FALSE
This is a fundamental misconception. Enzymes are catalysts, meaning they are not permanently altered during the reaction they facilitate. After the reaction is complete, the enzyme is free to catalyze the same reaction again with a new substrate molecule. The enzyme's structure remains largely unchanged throughout the process.
2. "All enzymes are proteins." FALSE
While the vast majority of enzymes are proteins, this statement is not universally true. As mentioned earlier, some RNA molecules possess catalytic activity and are referred to as ribozymes. These catalytic RNA molecules play essential roles in various cellular processes, including RNA splicing and protein synthesis. Therefore, the statement that all enzymes are proteins is inaccurate.
3. "Enzymes work equally well under all conditions." FALSE
Enzyme activity is highly dependent on environmental factors such as temperature and pH. Each enzyme has an optimal temperature and pH range within which it functions most effectively. Deviation from these optimal conditions can significantly reduce enzyme activity, or even cause irreversible denaturation (loss of its three-dimensional structure and hence, function). Extremes of temperature or pH can disrupt the weak bonds (hydrogen bonds, van der Waals forces) that maintain the enzyme's tertiary structure, leading to a loss of catalytic activity.
4. "Enzymes only speed up reactions; they don't change the equilibrium point." TRUE
This statement is accurate. Enzymes accelerate the rate at which a reaction reaches equilibrium but do not affect the equilibrium constant itself. Equilibrium refers to the point where the rates of the forward and reverse reactions are equal. Enzymes equally speed up both the forward and reverse reactions, thus not altering the final equilibrium concentrations of reactants and products.
5. "Enzymes are always highly specific and only interact with one substrate." FALSE
While many enzymes demonstrate high specificity, interacting with only one or a very limited range of substrates, some enzymes exhibit broader specificity. Some enzymes can act on a range of structurally related substrates, a phenomenon known as group specificity. Others may have multiple active sites, capable of interacting with different substrates simultaneously or sequentially. The degree of specificity varies significantly depending on the enzyme's structure and function.
6. "Enzyme activity is never regulated." FALSE
Enzyme activity is meticulously regulated within cells to maintain homeostasis and respond to changing environmental conditions. Regulation mechanisms can involve:
- Allosteric regulation: Binding of a molecule at a site other than the active site affects enzyme activity.
- Covalent modification: Chemical modification of the enzyme (e.g., phosphorylation) alters its activity.
- Feedback inhibition: The end product of a metabolic pathway inhibits an earlier enzyme in the pathway.
- Enzyme concentration: The amount of enzyme present can be regulated through gene expression.
7. "Once an enzyme is denatured, it can always be reactivated." FALSE
Denaturation involves the disruption of the enzyme's three-dimensional structure, leading to a loss of its catalytic activity. While some denaturation is reversible under mild conditions (e.g., slight changes in temperature or pH), severe denaturation, often caused by extreme conditions, is typically irreversible. The enzyme's structure is permanently altered, and its function cannot be restored.
8. "All enzyme inhibitors are irreversible." FALSE
Enzyme inhibitors are molecules that reduce or eliminate enzyme activity. They can be reversible or irreversible.
-
Reversible inhibitors: These bind to the enzyme non-covalently and can be removed, allowing the enzyme to regain its activity. Competitive inhibitors compete with the substrate for the active site, while non-competitive inhibitors bind at a different site, altering the enzyme's shape and reducing its activity.
-
Irreversible inhibitors: These bind to the enzyme covalently, permanently inactivating it. They often modify essential amino acid residues within the active site.
Conclusion: The Importance of Understanding Enzyme Properties
This in-depth analysis clarifies several common misunderstandings regarding enzyme function and behavior. Understanding the nuances of enzyme activity – their specificity, regulation, and environmental sensitivity – is crucial across various scientific disciplines. From drug design and development to the optimization of industrial processes involving biocatalysis, a precise understanding of these remarkable biological molecules is paramount. The statements that are not true about enzymes highlight the complexity and subtle variations in their properties, emphasizing the need for continuous research and a nuanced perspective on their biological roles. By dispelling these common misconceptions, we can foster a more accurate and comprehensive understanding of enzymes and their pivotal role in life's intricate processes.
Latest Posts
Latest Posts
-
Balanced Equation For H2so4 And Naoh
Mar 18, 2025
-
What Does 1 1 Ratio Mean
Mar 18, 2025
-
What Is 64 As A Fraction
Mar 18, 2025
-
The Way Of The World Analysis
Mar 18, 2025
-
Which Layer Of The Earth Is The Thinnest
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Statements Is Not True About Enzymes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
