Balanced Equation For H2so4 And Naoh
News Leon
Mar 18, 2025 · 6 min read
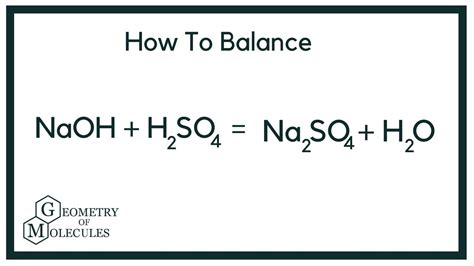
Table of Contents
The Balanced Equation for H₂SO₄ and NaOH: A Comprehensive Guide
The reaction between sulfuric acid (H₂SO₄) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction, a fundamental concept in chemistry. Understanding this reaction, including its balanced equation, is crucial for various applications, from titrations to industrial processes. This comprehensive guide will delve into the intricacies of this reaction, exploring the balanced equation, the stoichiometry involved, the different types of reactions that can occur, and the practical implications of understanding this seemingly simple chemical interaction.
Understanding the Reactants: H₂SO₄ and NaOH
Before diving into the reaction itself, let's briefly review the properties of the reactants: sulfuric acid and sodium hydroxide.
Sulfuric Acid (H₂SO₄)
Sulfuric acid is a strong diprotic acid, meaning it can donate two protons (H⁺ ions) per molecule. This characteristic significantly influences the reaction with a base like sodium hydroxide. It's a highly corrosive and reactive substance, widely used in various industrial processes, including fertilizer production, petroleum refining, and metal processing. Its strong acidic nature makes it crucial to handle it with appropriate safety precautions.
Sodium Hydroxide (NaOH)
Sodium hydroxide, commonly known as caustic soda or lye, is a strong base. This means it readily dissociates in water to release hydroxide ions (OH⁻). It's a highly alkaline substance used in numerous applications, ranging from drain cleaners and soap manufacturing to the production of various chemicals. Similar to sulfuric acid, sodium hydroxide requires careful handling due to its corrosive nature.
The Balanced Equation: Different Scenarios
The reaction between H₂SO₄ and NaOH isn't always straightforward. The balanced equation depends on the stoichiometric ratio of the reactants—the relative amounts of acid and base involved. Two main scenarios are possible:
Scenario 1: Complete Neutralization (1:2 Mole Ratio)
This is the most common scenario, where a sufficient amount of sodium hydroxide is added to neutralize both protons of sulfuric acid. The balanced equation for this complete neutralization is:
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l)
In this equation:
- H₂SO₄(aq) represents aqueous sulfuric acid. The (aq) indicates that it's dissolved in water.
- 2NaOH(aq) represents two moles of aqueous sodium hydroxide. The coefficient 2 is crucial for balancing the equation, ensuring that the number of atoms of each element is equal on both sides.
- Na₂SO₄(aq) represents aqueous sodium sulfate, the salt formed as a product of the neutralization reaction.
- 2H₂O(l) represents two moles of liquid water. The (l) indicates that it's in the liquid phase.
This equation demonstrates the classic acid-base neutralization: the hydrogen ions (H⁺) from the acid combine with the hydroxide ions (OH⁻) from the base to form water, while the remaining ions form the salt, sodium sulfate.
Scenario 2: Partial Neutralization (1:1 Mole Ratio)
If less sodium hydroxide is used, only one proton from the sulfuric acid will be neutralized. This results in the formation of sodium bisulfate (also known as sodium hydrogen sulfate), an acidic salt. The balanced equation for this partial neutralization is:
H₂SO₄(aq) + NaOH(aq) → NaHSO₄(aq) + H₂O(l)
This reaction produces sodium bisulfate (NaHSO₄), which still possesses an acidic proton. This scenario highlights the diprotic nature of sulfuric acid and the possibility of forming different products depending on the reactant ratios.
Stoichiometry and Calculations
Understanding the stoichiometry of the reaction allows us to perform quantitative calculations. Let's consider an example using the complete neutralization scenario:
Problem: How many grams of NaOH are required to completely neutralize 100 grams of H₂SO₄?
Solution:
-
Find the molar mass of each compound:
- H₂SO₄: (2 x 1.01) + (32.07) + (4 x 16.00) = 98.09 g/mol
- NaOH: 22.99 + 16.00 + 1.01 = 40.00 g/mol
-
Calculate the moles of H₂SO₄:
- Moles of H₂SO₄ = (100 g) / (98.09 g/mol) = 1.02 moles
-
Use the stoichiometric ratio from the balanced equation:
- From the balanced equation (H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l)), we see that 1 mole of H₂SO₄ reacts with 2 moles of NaOH.
-
Calculate the moles of NaOH required:
- Moles of NaOH = 1.02 moles H₂SO₄ x (2 moles NaOH / 1 mole H₂SO₄) = 2.04 moles NaOH
-
Calculate the grams of NaOH required:
- Grams of NaOH = 2.04 moles NaOH x 40.00 g/mol = 81.6 g
Therefore, 81.6 grams of NaOH are required to completely neutralize 100 grams of H₂SO₄. This type of calculation is vital in various analytical chemistry procedures, such as titrations.
Titration: A Practical Application
Titration is a common laboratory technique used to determine the concentration of an unknown solution using a solution of known concentration. The reaction between H₂SO₄ and NaOH is frequently used in titrations. A known volume of H₂SO₄ is titrated against a standardized NaOH solution until the equivalence point (complete neutralization) is reached. This point is often determined using an indicator, such as phenolphthalein, which changes color at the neutral pH. The volume of NaOH used allows us to calculate the concentration of the sulfuric acid.
Safety Precautions
Both sulfuric acid and sodium hydroxide are corrosive substances. Appropriate safety measures are crucial when handling these chemicals:
- Eye protection: Always wear safety goggles.
- Gloves: Use chemically resistant gloves.
- Lab coat: Wear a lab coat to protect your clothing.
- Ventilation: Work in a well-ventilated area or under a fume hood.
- Neutralization of spills: In case of spills, neutralize the acid or base carefully using the appropriate neutralizing agent and follow proper cleanup procedures. Never mix large quantities without proper safety equipment and training.
Ignoring these precautions can lead to severe burns and other injuries.
Beyond the Basics: Further Considerations
The reaction between H₂SO₄ and NaOH, while seemingly simple, offers opportunities for exploring more advanced chemical concepts:
- Heat of Neutralization: This reaction is exothermic, meaning it releases heat. Measuring the heat released can provide insights into the enthalpy change of the reaction.
- pH Changes: Monitoring the pH throughout the titration provides a detailed understanding of the changes in acidity and basicity.
- Conductivity Changes: Measuring the electrical conductivity of the solution during the titration allows tracking the changes in ion concentration.
- Equilibrium Considerations: While strong acids and bases react completely, a more nuanced understanding requires considering equilibrium concepts, particularly when dealing with weaker acids or bases.
Conclusion
The balanced equation for the reaction between sulfuric acid and sodium hydroxide is a fundamental concept with various applications across different scientific fields and industries. Understanding the different scenarios, stoichiometry, safety precautions, and related concepts provides a solid foundation for comprehending more complex chemical processes. This comprehensive guide aims to offer a detailed yet accessible understanding of this important chemical reaction, paving the way for further exploration into the fascinating world of chemistry. Remember always to prioritize safety when working with these chemicals.
Latest Posts
Latest Posts
-
Receptacle Is Part Of The Four Whorls
Mar 19, 2025
-
The Bending Of Waves Around A Barrier
Mar 19, 2025
-
A Surveyor Measures The Distance Across A Straight River
Mar 19, 2025
-
Fluid Pressure Against A Wall Or Cell Membranes Is Called
Mar 19, 2025
-
How Many Micrograms In A Kilogram
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For H2so4 And Naoh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
