Which Of The Following Pairs Of Ions Represent Isoelectronic Species
News Leon
Mar 17, 2025 · 5 min read
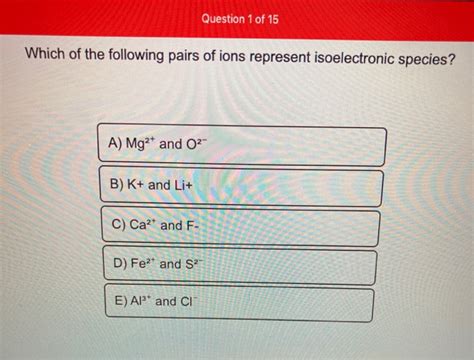
Table of Contents
Which of the Following Pairs of Ions Represent Isoelectronic Species? A Deep Dive into Isoelectronic Relationships
Understanding isoelectronic species is crucial in chemistry, offering insights into atomic structure, chemical bonding, and periodic trends. This article provides a comprehensive exploration of isoelectronic species, focusing on how to identify them, their properties, and the underlying principles that govern their behavior. We'll tackle the question directly, examining various ion pairs and determining which qualify as isoelectronic, then delve into the broader implications of this concept.
What are Isoelectronic Species?
Isoelectronic species are atoms or ions that possess the same number of electrons. This means they share an identical electronic configuration, although they may differ in their nuclear charge (number of protons). This shared electron configuration significantly influences their chemical and physical properties, often leading to similarities in size, ionization energy, and electron affinity. It's important to distinguish between isoelectronic species and isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. Isoelectronic species, on the other hand, can be atoms of different elements or ions derived from different elements.
Identifying Isoelectronic Species: A Step-by-Step Approach
Identifying isoelectronic species involves a methodical approach:
-
Determine the number of electrons in each atom or ion: This requires knowledge of the atomic number (number of protons) and the charge of the ion. Remember that a positive charge indicates the loss of electrons, while a negative charge indicates the gain of electrons.
-
Compare the electron counts: If two or more species have the same number of electrons, they are isoelectronic.
-
Consider electronic configuration: While the number of electrons is the primary criterion, examining the electronic configuration can provide additional insights into the similarities and differences in their properties.
Examples of Isoelectronic Species
Let's explore some examples to solidify our understanding:
-
O²⁻ and Ne: Oxygen (O) has an atomic number of 8, meaning 8 electrons in a neutral atom. The O²⁻ ion has gained two electrons, giving it a total of 10 electrons. Neon (Ne) also has an atomic number of 10, meaning 10 electrons. Therefore, O²⁻ and Ne are isoelectronic.
-
Na⁺ and Ne: Sodium (Na) has 11 electrons. Na⁺, having lost one electron, possesses 10 electrons, making it isoelectronic with Ne and O²⁻.
-
Mg²⁺, Na⁺, and Ne: Magnesium (Mg) has 12 electrons. Mg²⁺, having lost two electrons, has 10 electrons, making it isoelectronic with Na⁺ and Ne.
-
F⁻ and Ne: Fluorine (F) has 9 electrons. F⁻, having gained one electron, has 10 electrons, making it isoelectronic with Ne, Na⁺, Mg²⁺, and O²⁻.
These examples highlight that isoelectronic species can be formed by both cations (positively charged ions) and anions (negatively charged ions). The key is the identical number of electrons.
Analyzing Specific Ion Pairs for Isoelectronic Relationships
To address the original prompt directly, without specific ion pairs listed, let's create and analyze some potential pairings:
Pair 1: N³⁻ and O²⁻
- Nitrogen (N) has 7 electrons. N³⁻ gains 3 electrons, resulting in 10 electrons.
- Oxygen (O) has 8 electrons. O²⁻ gains 2 electrons, resulting in 10 electrons.
Conclusion: N³⁻ and O²⁻ are isoelectronic.
Pair 2: K⁺ and Ca²⁺
- Potassium (K) has 19 electrons. K⁺ loses 1 electron, resulting in 18 electrons.
- Calcium (Ca) has 20 electrons. Ca²⁺ loses 2 electrons, resulting in 18 electrons.
Conclusion: K⁺ and Ca²⁺ are isoelectronic.
Pair 3: Cl⁻ and Ar
- Chlorine (Cl) has 17 electrons. Cl⁻ gains 1 electron, resulting in 18 electrons.
- Argon (Ar) has 18 electrons.
Conclusion: Cl⁻ and Ar are isoelectronic.
Pair 4: S²⁻ and Cl⁻
- Sulfur (S) has 16 electrons. S²⁻ gains 2 electrons, resulting in 18 electrons.
- Chlorine (Cl) has 17 electrons. Cl⁻ gains 1 electron, resulting in 18 electrons.
Conclusion: S²⁻ and Cl⁻ are isoelectronic.
Pair 5: Fe²⁺ and Co³⁺
- Iron (Fe) has 26 electrons. Fe²⁺ loses 2 electrons, resulting in 24 electrons.
- Cobalt (Co) has 27 electrons. Co³⁺ loses 3 electrons, resulting in 24 electrons.
Conclusion: Fe²⁺ and Co³⁺ are isoelectronic.
Properties and Trends of Isoelectronic Species
The identical electron configuration of isoelectronic species leads to predictable trends in their properties:
-
Ionic Radius: As the nuclear charge increases (more protons) in a series of isoelectronic species, the ionic radius decreases. This is because the increased positive charge pulls the electrons closer to the nucleus. For example, in the series N³⁻, O²⁻, F⁻, Ne, Ne has the smallest ionic radius.
-
Ionization Energy: Ionization energy, the energy required to remove an electron, generally increases with increasing nuclear charge in an isoelectronic series. This is due to the stronger attraction between the nucleus and electrons.
-
Electron Affinity: Electron affinity, the energy change associated with adding an electron, generally decreases with increasing nuclear charge in an isoelectronic series. The increased nuclear charge makes it less favorable to add an additional electron.
Isoelectronic Series and Periodic Trends
Isoelectronic series provide a valuable tool for understanding periodic trends. By comparing the properties of isoelectronic species, we gain a deeper understanding of how electron configuration and nuclear charge influence the chemical behavior of elements. For instance, comparing the ionization energies of isoelectronic species helps to explain the trends observed across a period in the periodic table.
Applications of Isoelectronic Species
The concept of isoelectronic species has practical applications in various fields:
-
Spectroscopy: Isoelectronic species often exhibit similar spectral lines, facilitating spectral analysis and identification of unknown substances.
-
Material Science: Understanding isoelectronic relationships can aid in designing new materials with specific properties. By carefully choosing isoelectronic substitutions, scientists can fine-tune the electronic and structural characteristics of materials.
-
Computational Chemistry: Isoelectronic species serve as valuable models in computational chemistry, providing simplified systems for studying complex chemical processes.
Conclusion: The Significance of Isoelectronic Relationships
The study of isoelectronic species offers a powerful approach to understanding the interplay between electron configuration and chemical properties. By recognizing and comparing isoelectronic species, we can gain valuable insights into atomic structure, periodic trends, and the behavior of ions. This concept has widespread applications across various scientific disciplines, highlighting its significance in our understanding of the chemical world. Remember, the key is the identical number of electrons, irrespective of the number of protons or neutrons. This shared characteristic profoundly impacts the properties of these species, making it a crucial concept in chemical studies. Careful analysis using the steps outlined above allows for precise determination of isoelectronic relationships.
Latest Posts
Latest Posts
-
Which Of The Following Is An Incorrect Match
Mar 17, 2025
-
A Negatively Charged Ion Is Called A
Mar 17, 2025
-
What Is 0 02 As A Percent
Mar 17, 2025
-
Complete An Equation For The Function Graphed Above
Mar 17, 2025
-
What Is The Correct Sequence Of Embryonic Stages
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Pairs Of Ions Represent Isoelectronic Species . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
