Which Of The Following Molecules Has The Shortest Bond Length
News Leon
Apr 06, 2025 · 5 min read
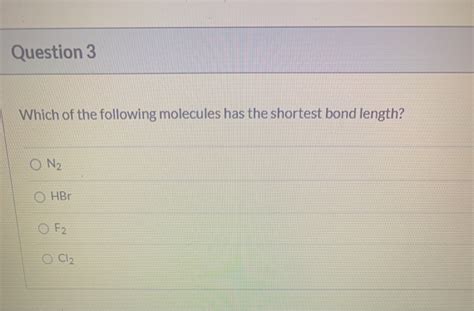
Table of Contents
Which of the Following Molecules Has the Shortest Bond Length? A Deep Dive into Bond Order and Atomic Radii
Determining which molecule possesses the shortest bond length requires a nuanced understanding of several key chemical concepts: bond order, atomic radii, and the interplay between electronegativity and hybridization. This article will explore these concepts in detail, providing a robust framework for predicting bond lengths and applying this knowledge to various molecular examples.
Understanding Bond Order
Bond order is a crucial factor influencing bond length. It represents the number of chemical bonds between a pair of atoms. A higher bond order indicates a stronger and shorter bond. Let's examine different bond orders:
-
Single Bond (Bond Order = 1): This involves one sigma (σ) bond, resulting in a relatively longer bond length. Examples include the C-C bond in ethane or the O-H bond in water.
-
Double Bond (Bond Order = 2): This comprises one sigma (σ) bond and one pi (π) bond. The presence of the additional π bond leads to a shorter and stronger bond compared to a single bond. Examples include the C=C bond in ethene or the C=O bond in formaldehyde.
-
Triple Bond (Bond Order = 3): This consists of one sigma (σ) bond and two pi (π) bonds. Triple bonds are the strongest and shortest due to the cumulative effect of three bonding interactions. Examples include the C≡C bond in ethyne (acetylene) or the N≡N bond in nitrogen gas.
Rule of Thumb: For molecules containing the same atoms, the higher the bond order, the shorter the bond length.
The Role of Atomic Radii
Atomic radii, or the size of an atom, also significantly influence bond length. Smaller atoms generally form shorter bonds. The trend in atomic radii follows periodic table trends:
-
Across a Period: Atomic radii generally decrease from left to right across a period due to increasing effective nuclear charge. Electrons are pulled closer to the nucleus, reducing the atomic size.
-
Down a Group: Atomic radii increase down a group due to the addition of electron shells.
Therefore, when comparing molecules with similar bond orders, smaller atoms will form shorter bonds.
Electronegativity's Influence
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A large difference in electronegativity between two bonded atoms can lead to a polar bond. While not directly affecting bond order, high electronegativity differences can slightly shorten bond lengths due to the stronger electrostatic attraction between the atoms. However, this effect is usually smaller than the influence of bond order and atomic radii.
Hybridization's Impact
Hybridization affects bond length indirectly by influencing the geometry and orbital overlap of the bond. Different hybridization states (sp, sp², sp³) lead to varying degrees of s-character in the hybrid orbitals. Higher s-character leads to shorter and stronger bonds because s orbitals are closer to the nucleus than p orbitals. For example, a C-C bond in an sp hybridized molecule (like ethyne) is shorter than in an sp³ hybridized molecule (like ethane).
Comparing Molecules: A Case Study
Let's consider a few hypothetical molecules to illustrate the principles discussed above:
Molecule A: A molecule with a triple bond between two small atoms (e.g., N₂). Molecule B: A molecule with a double bond between two slightly larger atoms (e.g., O₂). Molecule C: A molecule with a single bond between two larger atoms (e.g., Cl₂).
Based on our understanding of bond order and atomic radii, we can predict that Molecule A (N₂) would likely have the shortest bond length. It has the highest bond order (3) and involves relatively small atoms (nitrogen). Molecule B would have a longer bond length due to the lower bond order (2), and Molecule C would have the longest bond length due to the lowest bond order (1) and the largest atoms.
Note: This comparison is a simplification. In reality, the exact bond length is determined by complex quantum mechanical interactions. However, the principles outlined here provide a solid foundation for making reasonable predictions.
Advanced Considerations: Resonance and Delocalization
In some molecules, resonance and delocalization of electrons can influence bond lengths. Resonance structures depict different possible arrangements of electrons within a molecule, and the actual structure is a hybrid of these resonance forms. Delocalization of electrons across multiple atoms leads to an average bond order, often resulting in bond lengths intermediate between single and double bonds. For example, benzene has delocalized pi electrons, resulting in bond lengths that are equal and intermediate between typical C-C single and double bonds.
Computational Chemistry and Bond Length Determination
Accurate determination of bond lengths often relies on computational chemistry techniques. Methods like Density Functional Theory (DFT) and ab initio calculations provide highly accurate predictions of molecular structures and properties, including bond lengths. These computational methods are essential for complex molecules where simple rules of thumb may not suffice.
Conclusion: Predicting Bond Length
Predicting the shortest bond length among several molecules involves carefully considering the interplay of multiple factors. Bond order is often the most dominant factor. Higher bond order strongly implies shorter bond length. However, atomic radii play a significant role, especially when comparing molecules with similar bond orders. Smaller atoms result in shorter bonds. Electronegativity and hybridization have secondary influences, subtly affecting bond lengths through polarization and orbital overlap. For complex molecules, resonance and advanced computational methods are crucial for accurate bond length determination. By carefully applying these principles, we can make informed predictions about relative bond lengths within a set of molecules. The ability to predict bond length is invaluable in various fields, including drug design, materials science, and understanding chemical reactivity. Remember that these are general rules and exceptions always exist.
Latest Posts
Latest Posts
-
Four Basic Functions Of A Computer
Apr 07, 2025
-
The Oxygen Released During Photosynthesis Comes From
Apr 07, 2025
-
Is Cellular Respiration A Chemical Change
Apr 07, 2025
-
What Is The Oxidation State Of Chlorine In Hclo2
Apr 07, 2025
-
What Ions Are Produced From Acids And From Bases
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Molecules Has The Shortest Bond Length . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
