Which Of The Following Is A Tertiary Amine
News Leon
Mar 17, 2025 · 6 min read
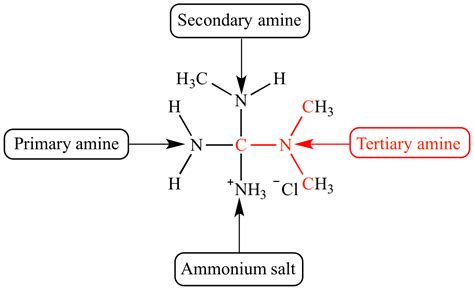
Table of Contents
Which of the Following is a Tertiary Amine? A Deep Dive into Amine Classification
Understanding amines and their classification is fundamental in organic chemistry. Amines are organic compounds derived from ammonia (NH₃) by replacing one or more hydrogen atoms with alkyl or aryl groups. This seemingly simple substitution leads to a diverse range of compounds with varying properties and reactivities. This article will delve deep into amine classification, specifically focusing on tertiary amines, and explore how to identify them amongst other amine types.
Understanding Amine Classification: Primary, Secondary, and Tertiary Amines
The classification of amines is primarily based on the number of alkyl or aryl groups attached to the nitrogen atom. This simple distinction defines three major categories:
Primary Amines (1°)
Primary amines have only one alkyl or aryl group attached to the nitrogen atom. The general formula is R-NH₂, where 'R' represents the alkyl or aryl group. Examples include methylamine (CH₃NH₂) and aniline (C₆H₅NH₂).
Secondary Amines (2°)
Secondary amines have two alkyl or aryl groups attached to the nitrogen atom. The general formula is R¹-NH-R², where 'R¹' and 'R²' represent alkyl or aryl groups, which can be the same or different. Dimethylamine ((CH₃)₂NH) and N-methylaniline (C₆H₅NHCH₃) are examples.
Tertiary Amines (3°)
Tertiary amines have three alkyl or aryl groups attached to the nitrogen atom. The general formula is R¹-N-R²-R³, where 'R¹', 'R²', and 'R³' represent alkyl or aryl groups, which can be the same or different. Trimethylamine ((CH₃)₃N) and N,N-dimethylaniline (C₆H₅N(CH₃)₂) are classic examples.
Identifying Tertiary Amines: A Step-by-Step Approach
Identifying a tertiary amine requires a careful examination of the molecular structure. Here's a systematic approach:
-
Locate the Nitrogen Atom: The first step is to identify the nitrogen atom within the molecule. Nitrogen is usually represented by the symbol 'N'.
-
Count the Attached Groups: Count the number of alkyl or aryl groups directly bonded to the nitrogen atom. Ignore any other atoms or groups attached to these alkyl or aryl groups.
-
Classify Based on the Count:
- One group: Primary amine (1°)
- Two groups: Secondary amine (2°)
- Three groups: Tertiary amine (3°)
Let's illustrate this with examples:
Example 1: Consider the molecule (CH₃)₃N.
- Step 1: The nitrogen atom is clearly visible.
- Step 2: Three methyl (CH₃) groups are directly attached to the nitrogen.
- Step 3: Since three groups are attached, this is a tertiary amine.
Example 2: Consider the molecule CH₃NHCH₂CH₃.
- Step 1: The nitrogen atom is present.
- Step 2: Two alkyl groups are attached to the nitrogen: one methyl (CH₃) and one ethyl (CH₂CH₃).
- Step 3: With two groups, this is a secondary amine.
Example 3: Consider a more complex molecule, such as N,N-diethyl-m-toluidine. To simplify, let's represent the m-toluidine group as 'Tol'.
- Step 1: Locate the nitrogen atom.
- Step 2: Three groups are attached to the nitrogen: two ethyl groups (Et) and one m-toluidine group (Tol).
- Step 3: This is a tertiary amine.
Differentiating Tertiary Amines from Other Amine Types: Key Distinctions
While the counting method is straightforward, understanding the subtle differences between the amine types is crucial for comprehending their diverse chemical behavior. Here's a comparison highlighting key distinctions:
| Feature | Primary Amine (1°) | Secondary Amine (2°) | Tertiary Amine (3°) |
|---|---|---|---|
| Number of alkyl/aryl groups | One | Two | Three |
| Hydrogen atoms on nitrogen | Two | One | Zero |
| H-bonding ability | Strong | Moderate | Weak or Absent |
| Solubility in water | Generally more soluble | Less soluble than 1° | Least soluble |
| Boiling point | Relatively high | Higher than 1° | Lower than 1° and 2° |
| Basicity | Moderately basic | Less basic than 1° | Least basic |
The differences in hydrogen bonding ability significantly impact their physical properties. Primary amines, with two hydrogen atoms on the nitrogen, can form strong hydrogen bonds with water, leading to higher solubility. Tertiary amines, lacking hydrogen atoms on nitrogen, exhibit weaker hydrogen bonding and therefore lower solubility. Similarly, boiling points are influenced by the strength of intermolecular forces, with primary amines typically having higher boiling points due to strong hydrogen bonding.
The Role of Steric Hindrance in Tertiary Amines
The presence of three bulky alkyl or aryl groups in tertiary amines leads to significant steric hindrance. This steric hindrance affects several aspects of their reactivity:
-
Nucleophilicity: Steric hindrance reduces the accessibility of the nitrogen lone pair, making tertiary amines less nucleophilic than primary or secondary amines.
-
Reactivity with alkyl halides: Tertiary amines are less reactive towards alkyl halides in alkylation reactions due to steric hindrance.
-
Acylation: Similar to alkylation, steric hindrance reduces the reactivity of tertiary amines towards acylating agents.
Understanding steric hindrance is crucial for predicting the reactivity of tertiary amines in various chemical reactions.
Applications of Tertiary Amines
Tertiary amines find widespread applications in various fields due to their unique properties:
-
Pharmaceuticals: Many pharmaceutical drugs contain tertiary amine moieties, contributing to their biological activity and interaction with receptors.
-
Polymer Chemistry: Tertiary amines act as catalysts and initiators in various polymerization reactions.
-
Solvents: Certain tertiary amines, due to their polarity and ability to form hydrogen bonds, serve as excellent solvents in chemical reactions.
-
Surfactants: Some tertiary amines are used as surfactants, reducing surface tension and improving wetting properties.
Advanced Topics and Further Exploration
The world of amines extends beyond the basic classification. Here are some areas for further exploration:
-
Heterocyclic Amines: These are amines where the nitrogen atom is part of a ring structure. Many biologically important molecules, such as alkaloids, belong to this category.
-
Quaternary Ammonium Salts: These are formed by the addition of a fourth alkyl or aryl group to the nitrogen atom of a tertiary amine, resulting in a positively charged species. These salts have various applications, such as in detergents and disinfectants.
-
Amine Reactivity and Synthesis: A comprehensive study of amine reactivity, encompassing reactions like alkylation, acylation, and diazotization, provides a deeper understanding of their versatile chemical behavior and synthetic utility.
Conclusion: Mastering Amine Classification for Success in Organic Chemistry
Accurately identifying tertiary amines, alongside primary and secondary amines, forms a cornerstone of organic chemistry. Understanding their classification, based on the number of alkyl or aryl groups attached to the nitrogen atom, is crucial for predicting their properties and reactivity. By mastering this fundamental concept and exploring the more advanced topics, you'll build a strong foundation in organic chemistry. The detailed examples and comparative analysis provided in this article aim to equip you with the knowledge and skills needed to confidently identify tertiary amines and appreciate their importance in various chemical applications. Remember to practice identifying amines in different molecular structures to solidify your understanding.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Tertiary Amine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
