Which Of The Following Are Meso Compounds
News Leon
Mar 26, 2025 · 5 min read
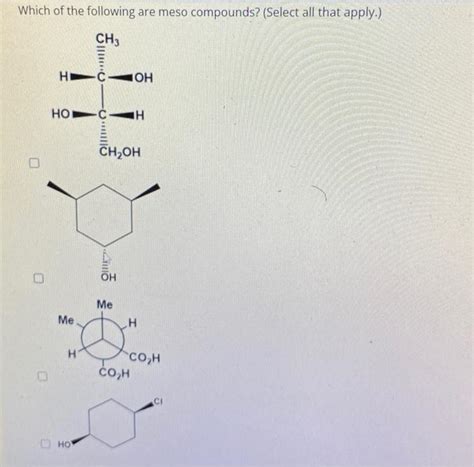
Table of Contents
Which of the Following are Meso Compounds? A Comprehensive Guide
Meso compounds represent a fascinating area within stereochemistry, often causing confusion even for seasoned organic chemists. Understanding what constitutes a meso compound is crucial for predicting and interpreting the behavior of molecules, especially in reactions involving chiral centers. This in-depth guide will clarify the concept of meso compounds, detailing their characteristics, differentiating them from other stereoisomers, and providing numerous examples to solidify your understanding.
Understanding Chirality and Stereoisomers
Before diving into meso compounds, let's revisit the fundamental concepts of chirality and stereoisomers.
Chirality: The Handedness of Molecules
Chirality refers to a molecule's "handedness"—its inability to be superimposed on its mirror image. This is usually due to the presence of one or more chiral centers, also known as stereocenters. A chiral center is typically a carbon atom bonded to four different groups. Molecules with chiral centers can exist as enantiomers, which are non-superimposable mirror images, much like your left and right hands.
Stereoisomers: Beyond Enantiomers
Stereoisomers are molecules with the same molecular formula and connectivity but different spatial arrangements of atoms. Enantiomers are one type of stereoisomer, but there's another crucial category: diastereomers. Diastereomers are stereoisomers that are not mirror images of each other. Meso compounds are a specific subset of diastereomers.
Defining Meso Compounds: Internal Compensation of Chirality
A meso compound is a molecule with two or more chiral centers that is achiral as a whole. This seemingly paradoxical situation arises because of internal compensation of chirality. While individual chiral centers possess chirality, the molecule's overall symmetry cancels out this chirality, making it superimposable on its mirror image.
Key characteristics of meso compounds:
- At least two chiral centers: A single chiral center always leads to chirality.
- Internal plane of symmetry: This is the crucial feature. A meso compound possesses a plane of symmetry that divides the molecule into two identical halves. This plane of symmetry effectively cancels out the chirality introduced by the individual chiral centers.
- Achiral: Despite having chiral centers, the overall molecule is achiral. It is superimposable on its mirror image.
- Optically inactive: Because it's achiral, a meso compound does not rotate plane-polarized light.
Distinguishing Meso Compounds from Other Stereoisomers
The key difference between meso compounds and other stereoisomers lies in the presence of an internal plane of symmetry and the resulting achirality. Let's compare:
| Feature | Meso Compound | Enantiomers | Diastereomers (excluding meso) |
|---|---|---|---|
| Chiral Centers | Two or more | One or more | One or more |
| Plane of Symmetry | Present | Absent | Absent (typically) |
| Chirality | Achiral (overall) | Chiral | Chiral (typically) |
| Optical Activity | Optically inactive | Optically active (equal and opposite rotation) | Optically active (different rotations) |
| Superimposition | Superimposable on its mirror image | Non-superimposable mirror images | Non-superimposable |
Identifying Meso Compounds: Practical Examples and Exercises
Let's examine several examples to illustrate the identification of meso compounds.
Example 1: Tartaric Acid
Tartaric acid, a molecule with two chiral centers, exists in three forms:
- (2R,3R)-Tartaric acid: A chiral enantiomer.
- (2S,3S)-Tartaric acid: The enantiomer of (2R,3R)-tartaric acid.
- (2R,3S)-Tartaric acid (meso-tartaric acid): This possesses an internal plane of symmetry, making it achiral and therefore a meso compound. Notice how the two chiral centers have opposite configurations.
Example 2: 2,3-Dibromobutane
2,3-Dibromobutane also illustrates the concept. The (2R,3R) and (2S,3S) isomers are enantiomers. However, the (2R,3S) isomer is a meso compound due to its internal plane of symmetry. Draw the structures to visualize the plane of symmetry.
Example 3: More Complex Molecules
Meso compounds can be more complex than simple four-carbon structures. Look for molecules with multiple chiral centers arranged symmetrically around a plane of symmetry. It's crucial to analyze the three-dimensional structure using models or advanced drawing techniques to confidently identify the presence of a plane of symmetry.
Practical Applications and Significance of Meso Compounds
Understanding meso compounds is not merely an academic exercise; it has important practical implications:
- Drug Design and Development: The chirality of molecules plays a vital role in their biological activity. Understanding whether a drug molecule is chiral or a meso compound is critical in predicting its efficacy and potential side effects.
- Material Science: The properties of materials, including polymers and crystals, are often influenced by the chirality of their constituent molecules. Meso compounds can exhibit unique material properties.
- Organic Synthesis: The synthesis and purification of chiral compounds often require careful consideration of potential meso compound formation. Understanding meso compounds helps chemists control stereochemical outcomes.
Advanced Considerations and Challenges
Identifying meso compounds can become challenging with larger, more complex molecules. Advanced techniques are sometimes needed:
- 3D Molecular Modeling: Software packages can create 3D models, allowing you to rotate and manipulate the molecule to easily visualize the presence of a plane of symmetry.
- Symmetry Operations: Formal symmetry operations can be used to rigorously determine if a molecule possesses a plane of symmetry.
- Analyzing Fischer Projections: Fischer projections can be helpful in identifying internal symmetry, particularly in simpler molecules.
Conclusion: Mastering the Art of Meso Compound Identification
Meso compounds are a fascinating aspect of stereochemistry, requiring a firm grasp of chirality, stereoisomers, and the crucial concept of internal compensation of chirality. Through careful analysis of molecular structure and symmetry, you can confidently identify and differentiate meso compounds from other stereoisomers. This understanding is vital not only for academic pursuits but also for applications in various scientific and technological fields. Remember, practice is key! Work through numerous examples to solidify your understanding and develop proficiency in identifying meso compounds. Utilize the tools mentioned above, and you'll quickly become adept at navigating this often-challenging but ultimately rewarding aspect of organic chemistry.
Latest Posts
Latest Posts
-
How Long Does It Take To Read 120 Pages
Mar 29, 2025
-
What Is An Example Of Tertiary Consumer
Mar 29, 2025
-
A Long Nonconducting Solid Cylinder Of Radius
Mar 29, 2025
-
What Will Happen If Ribosomes Are Removed From The Cell
Mar 29, 2025
-
How Many Hydrogen Bonds Between C And G
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Are Meso Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
