Which Molecule Contains Sp Hybridized Orbitals
News Leon
Mar 21, 2025 · 6 min read
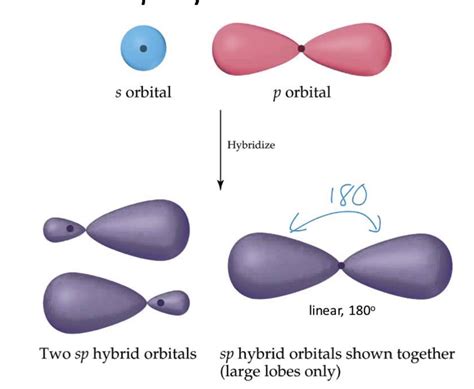
Table of Contents
Which Molecules Contain sp Hybridized Orbitals? A Comprehensive Guide
Understanding hybridization is crucial for grasping the shapes and properties of molecules. This detailed guide explores molecules containing sp hybridized orbitals, explaining the concept of hybridization, identifying key characteristics of sp hybridized atoms, and providing numerous examples.
What is Hybridization?
Hybridization is a theoretical concept in chemistry that explains the bonding in molecules more accurately than simple valence bond theory. It postulates that atomic orbitals within an atom combine to form new hybrid orbitals that are energetically more stable and better suited for bonding. This mixing of atomic orbitals occurs before bonding takes place. The resulting hybrid orbitals have different shapes and energies than the original atomic orbitals.
The type of hybridization depends on the number of atomic orbitals involved in the mixing process. For example, sp hybridization involves the mixing of one s orbital and one p orbital, resulting in two sp hybrid orbitals. Other common types include sp², involving one s and two p orbitals, and sp³, involving one s and three p orbitals.
Characteristics of sp Hybridized Atoms
Atoms exhibiting sp hybridization possess several key characteristics:
-
Linear Geometry: The two sp hybrid orbitals are oriented 180° apart, resulting in a linear molecular geometry around the central atom. This is a defining feature of sp hybridization.
-
Two Sigma Bonds: Each sp hybrid orbital forms a sigma (σ) bond with another atom. Sigma bonds are strong, single bonds formed by the direct overlap of orbitals.
-
Two Unhybridized p Orbitals: Two p orbitals remain unhybridized and are perpendicular to the plane formed by the sp hybrid orbitals. These unhybridized p orbitals are involved in the formation of pi (π) bonds.
-
Presence of Multiple Bonds: Molecules with sp hybridized atoms often contain multiple bonds (double or triple bonds), as the unhybridized p orbitals participate in pi bond formation.
Identifying sp Hybridized Atoms in Molecules
To identify sp hybridized atoms in a molecule, follow these steps:
-
Draw the Lewis Structure: This helps visualize the bonding and lone pairs of electrons.
-
Determine the Steric Number: The steric number is the sum of the number of sigma bonds and lone pairs around the central atom.
-
Relate Steric Number to Hybridization:
- Steric number of 2 indicates sp hybridization.
- Steric number of 3 indicates sp² hybridization.
- Steric number of 4 indicates sp³ hybridization.
-
Check for Linear Geometry: The atom must be in a linear arrangement with its bonded atoms.
Examples of Molecules with sp Hybridized Atoms
Let's explore various examples, focusing on the central atom's hybridization:
1. Acetylene (Ethyne, C₂H₂)
Acetylene is the simplest example. Each carbon atom has a steric number of 2 (one sigma bond to a carbon and one sigma bond to a hydrogen). Therefore, each carbon atom is sp hybridized. The molecule exhibits a linear geometry. The two carbon atoms are connected by a triple bond (one sigma and two pi bonds), with the pi bonds formed by the overlap of the unhybridized p orbitals on each carbon atom.
2. Carbon Dioxide (CO₂)
In CO₂, the carbon atom is bonded to two oxygen atoms with double bonds. The steric number of carbon is 2 (two sigma bonds, no lone pairs). Thus, the carbon atom in CO₂ is sp hybridized, and the molecule has a linear structure.
3. Hydrogen Cyanide (HCN)
HCN is another linear molecule. The carbon atom has a steric number of 2 (one sigma bond to hydrogen and one sigma bond to nitrogen). Consequently, the carbon atom is sp hybridized. The carbon and nitrogen atoms are connected by a triple bond.
4. Carbon Monoxide (CO)
Similar to the previous examples, the carbon atom in CO possesses a steric number of 2 (one sigma bond to oxygen and no lone pairs). Hence, the carbon is sp hybridized. The molecule is linear.
5. BeCl₂
Beryllium dichloride (BeCl₂) is a simple example with a central beryllium atom bonded to two chlorine atoms. The beryllium atom has a steric number of 2 (two sigma bonds, no lone pairs) and is therefore sp hybridized. The molecule adopts a linear structure.
6. Formaldehyde (CH₂O)
While the carbon atom in formaldehyde is not sp hybridized (it's sp²), understanding its hybridization contrasts with sp hybridized molecules. The carbon atom forms one double bond with oxygen and two single bonds with hydrogen, resulting in a steric number of 3 and sp² hybridization. This demonstrates the importance of carefully determining the steric number.
7. More Complex Examples: Alkynes
Alkynes are hydrocarbons containing carbon-carbon triple bonds. The carbon atoms involved in the triple bond are all sp hybridized. For example, 1-butyne (CH₃CH₂C≡CH) contains two sp hybridized carbon atoms within the triple bond.
8. Nitriles
Nitriles are organic compounds containing a cyano group (-C≡N). The carbon atom in the cyano group is sp hybridized due to the triple bond with nitrogen. Acetonitrile (CH₃CN) is a typical example.
9. Metal Carbonyls
Some metal carbonyl complexes also contain sp hybridized carbon atoms. For example, nickel tetracarbonyl (Ni(CO)₄) features carbon atoms in the carbonyl ligands that are sp hybridized.
10. Isocyanides
Isocyanides (R-N≡C) also contain sp hybridized carbon atoms due to the presence of the triple bond.
Beyond Simple Molecules: More Complex Scenarios
The concept of sp hybridization extends beyond simple diatomic and triatomic molecules. In more complex organic and inorganic compounds, identifying sp hybridized atoms requires careful analysis of the Lewis structure and the steric number of each atom. Remember that the presence of multiple bonds and linear geometries strongly suggests sp hybridization. However, always verify through the steric number calculation.
Exceptions and Limitations
While hybridization is a powerful tool for understanding molecular structure, it's important to recognize its limitations:
-
It's a Model: Hybridization is a theoretical model that simplifies the complex interactions between electrons and atomic nuclei.
-
Not all Bonds are Perfectly Hybridized: The extent of hybridization can vary, depending on the specific atoms and bonds involved.
-
Limitations in Complex Molecules: Applying hybridization theory to very large or complex molecules can become challenging.
Conclusion
Understanding sp hybridization is fundamental to comprehending the structures and properties of a wide range of molecules. By systematically determining the steric number and identifying linear geometries around atoms, one can accurately identify sp hybridized atoms. This knowledge is vital for predicting molecular shapes, bond angles, and chemical reactivity. This guide provides a solid foundation for further exploration of this important chemical concept. Remember to practice identifying sp hybridized atoms in various molecules to solidify your understanding. The more examples you work through, the more comfortable you'll become with applying this valuable theoretical tool.
Latest Posts
Latest Posts
-
Nucleotides Contain A Phosphate A Sugar And A Nitrogenous
Mar 28, 2025
-
In A Mixed Market Economy Property Owned By The Government
Mar 28, 2025
-
A Solution Of Substance X Is Used For Whitewashing
Mar 28, 2025
-
Is Air A Mixture Or A Compound
Mar 28, 2025
-
Write The Balanced Chemical Equation For Photosynthesis
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Molecule Contains Sp Hybridized Orbitals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
